Chemistry
Choose from the list of substances — Acetylene gas, aqua fortis, coke, brass, barium chloride, bronze, platinum.
An aqueous salt solution used for testing sulphate radical.
Related Questions
Mention the colour change when following indicators are added:
Solution Acids Alkalies (a) Alkaline phenolphthalein solution, (b) Methyl orange solution, (c) Neutral litmus solution (a) Select the correct answer from A, B, C and D.
A. Nitroso iron (II) sulphate
B. Iron (III) chloride
C. Chromium sulphate
D. Lead (II) chloride.
(i) The compound which is responsible for the green colour formed when SO2 is bubbled through acidified potassium dichromate solution.
(ii) Compound responsible for brown ring.
Identify :
(a) The flame test with a salt P gave a brick red flame. What is the cation in P.
(b) pH of liquid R is 10. What kind of substance is R ?
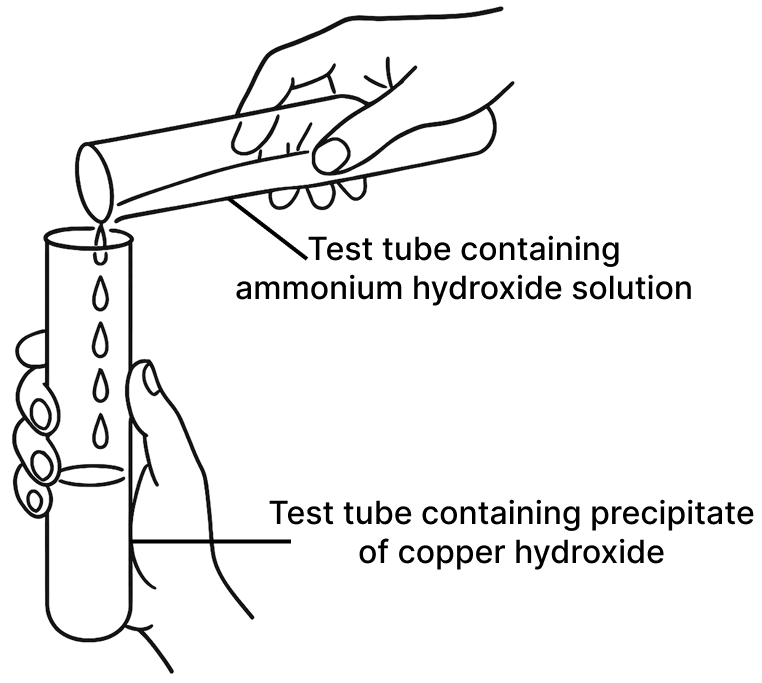
A student was asked to perform two experiments in the laboratory based on the instructions given:
Observe the picture given below and state one observation for each of the Experiments 1 and 2 that you would notice on mixing the given solutions.
(a) Experiment 1
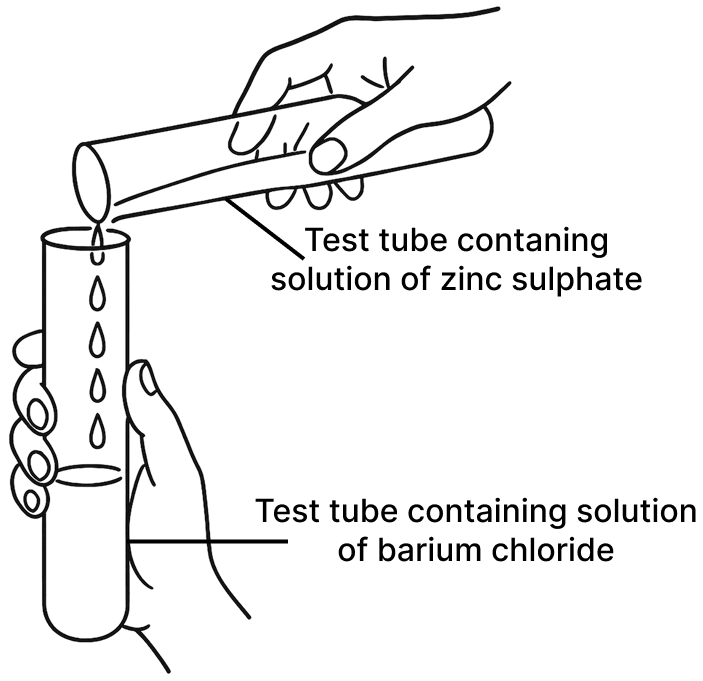
(b) Experiment 2