Chemistry
Distinguish between the following as directed:
(a) Sodium Sulphite and Sodium Sulphate by using dilute HCl.
(b) Ammonium chloride and Sodium chloride by using Calcium hydroxide.
(c) Lead nitrate and silver nitrate by using HCl.
Answer
(a) When Sodium sulphite reacts with HCl, effervescence occurs and a pungent gas (SO2) is evolved that turns acidified potassium dichromate paper from orange to green.
Na2SO3 + 2HCl ⟶ 2NaCl + H2O + SO2 ↑
Whereas, Sodium sulphate (Na2SO4) gives no visible reaction with HCl and no gas is evolved.
(b) Ammonium Chloride (NH4Cl) reacts with Ca(OH)2 to release ammonia gas (NH3) which has pungent smell, turns red litmus blue.
2NH4Cl + Ca(OH)2 ⟶ CaCl2 + 2H2O + 2NH3↑
Whereas, sodium chloride shows no reaction with Calcium hydroxide.
(c) Lead Nitrate (Pb(NO3)2) reacts with HCl, forms a white precipitate of lead chloride (PbCl2) which is soluble in hot water.
Pb(NO3)2 + 2HCl ⟶ PbCl2 ↓ + 2HNO3
However, when Silver nitrate reacts with HCl, it forms a white precipitate of silver chloride (AgCl) which is insoluble in hot water
AgNO3 + HCl ⟶ AgCl ↓ + HNO3
Related Questions
An organic compound contains: H = 6.32 %, N = 17.76%. In the vapour state, this compound is 39.5 times as heavy as the same volume of hydrogen.
(a) Find the molecular formula of the compound. (At wt: H = 1 N = 14 )
(b) Calculate the number of hydrogen atoms in one mole of this compound.
An element X has atomic number 12. Answer the following questions:
(a) State the period and group to which it belongs.
(b) Write the formula of the compound formed between X and the second member of the halogen group
A basic gas is dissolved in water. Draw the electron dot diagram of the ions formed.
The formulae of some organic compounds are:
A

B

C

D
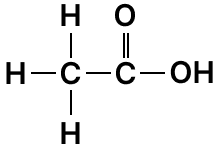
(a) Write an equation for the preparation of A using B.
(b) Name the compound formed when B and D reacts in presence of a mineral acid.
(c) Write an equation for the preparation of C.