Chemistry
In an experiment done with a burning candle, the apparatus was set up as shown below. Initially, the candle burns but after sometime it gets extinguished.
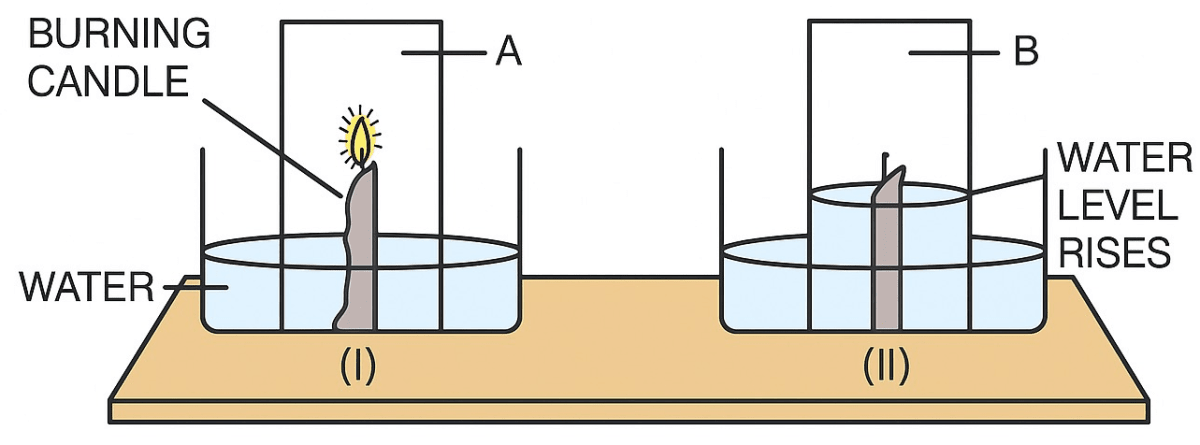
Look at the diagram and answer the questions given below.
(a) Label A and B.
(b) Why does the candle burn in (I) but gets extinguished in (II) ?
(c) Why does the water level rise after sometime ?
(d) What conclusion is drawn by the experiment show above ?
Answer
(a) In the given diagram A and B are:
A → Air
B → Inactive air
(b) The candle burn in (I) because of the presence of 'active air' that contains oxygen, which is used by the candle to burn. The candle does not burn in case of (II), because the jar consist of 'inactive air', consist mainly nitrogen, which does not support burning.
(c) When the candle is lit in the jar, it starts burning using the active air present in the jar, upto one-fifth part of the jar containing oxygen helps the candle to burn. After sometime the candle gets extinguished and the water level rises upto one-fifth part of the jar to compensate the loss of oxygen.
(d) This proves that air contains both oxygen and nitrogen. It clearly explains that air is made up of an 'active' part and an 'inactive' part. The 'active' part of air, which is used up when substances burn, is about one-fifth of the total volume of the air. It is a gas called oxygen. The 'inactive' part, which does not support burning, consists mainly of a gas called nitrogen, constituting 4/5th of air by volume.