Physics
30 g of ice at 0°C is used to bring down the temperature of a certain mass of water at 70°C to 20°C. Find the mass of water.
[Specific heat capacity of water = 4.2 J g⁻¹ °C⁻¹ and specific latent heat of ice = 336 J g⁻¹]
Related Questions
Study the diagram given below and answer the questions that follow :
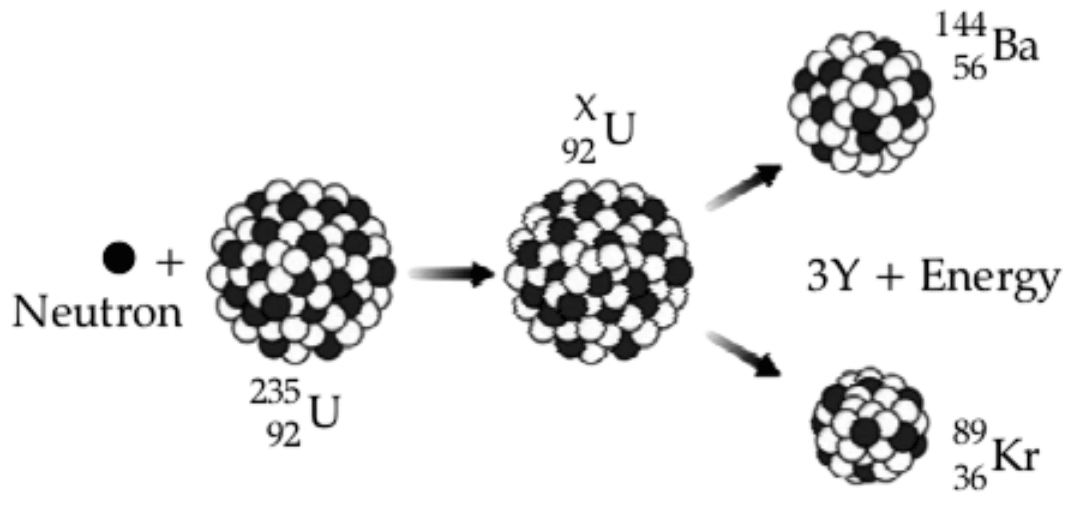
(a) Name the process depicted in the diagram.
(b) What is the value of X?
(c) Identify Y, the missing product of the reaction.
Three identical bulbs B1, B2 and B3 each of power rating 18 W, 12 V are connected to a battery of 12 V.
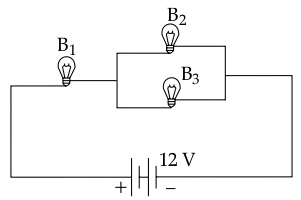
(a) Calculate :
- the resistance of each bulb
- the current drawn from the cell
(b) If the bulb B3 is removed from the circuit, then will the brightness of the bulb B1 increase, decrease or remain the same?
(a) A certain amount of heat will warm 1 g of material X by 10°C and 1 g of material Y by 40°C. Which material has higher specific heat capacity?
(b) Which material, X or Y, would you select to make a calorimeter?
(c) The specific heat capacity of a substance remains the same when it changes its state from solid to liquid. State True or False.
A copper rod PQ carrying current is kept in a magnetic field as shown in the diagram.
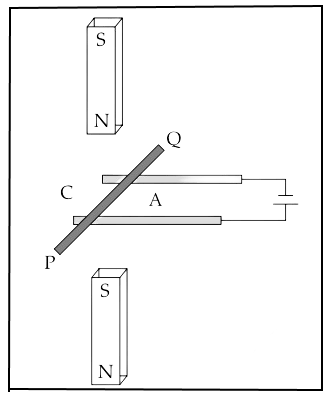
(a) The copper rod PQ will move towards C. State True or False.
(b) Name the law used to determine the direction of motion of PQ.
(c) What will be the effect on the force experienced, if the rod PQ is replaced by another copper rod of same length but of greater cross-sectional area?
(d) Justify your answer in (c).