Chemistry
The drying agent used to dry Ammonia is:
- Concentrated Sulphuric acid
- Calcium oxide
- Sulphurous acid
- Calcium hydroxide
Answer
Calcium oxide
Reason — The drying agent used to dry Ammonia is quicklime or calcium oxide (CaO). Other drying agents like conc. sulphuric acid and sulphurous acid are not used, as ammonia being basic, reacts with them. Calcium hydroxide (slaked lime) is slightly basic, but less effective than calcium oxide in drying. It may also partially react with ammonia.
Related Questions
The hydroxide which is soluble in excess of NH4OH is:
- Ferric hydroxide
- Lead hydroxide
- Copper hydroxide
- Calcium hydroxide
The main components of brass are:
- Copper and tin
- Copper and iron
- Copper and lead
- Copper and zinc
The percentage of nitrogen present in urea (NH2)2CO is: [R.A.M. of N = 14, C= 12, O = 16, H = 1]
- 23.36
- 46.67
- 19.35
- 43.87
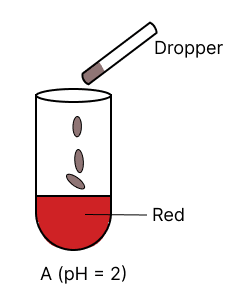
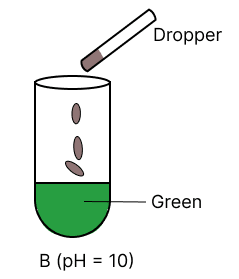
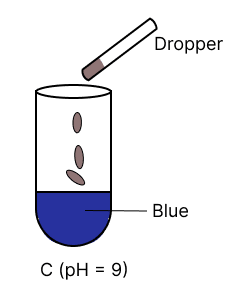
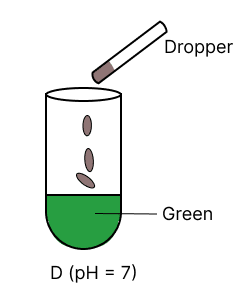
A few drops of universal indicator are added to colourless solution A, B, C and D with pH 2, 10, 9 and 7 respectively. Which of the following test tube is labelled with incorrect colour ?