Chemistry
The percentage of nitrogen present in urea (NH2)2CO is: [R.A.M. of N = 14, C= 12, O = 16, H = 1]
- 23.36
- 46.67
- 19.35
- 43.87
Stoichiometry
3 Likes
Answer
46.67
Reason —
Molar mass of urea (CON2H4) = 12 + 16 + 28 + 4 = 60 g
Molar mass of nitrogen (N2) = 2 x 14 = 28 g
60 g urea has mass of nitrogen = 28 g
∴ 100 g urea will have mass
=
= 46.67%
Answered By
2 Likes
Related Questions
The main components of brass are:
- Copper and tin
- Copper and iron
- Copper and lead
- Copper and zinc
The drying agent used to dry Ammonia is:
- Concentrated Sulphuric acid
- Calcium oxide
- Sulphurous acid
- Calcium hydroxide
A few drops of universal indicator are added to colourless solution A, B, C and D with pH 2, 10, 9 and 7 respectively. Which of the following test tube is labelled with incorrect colour ?
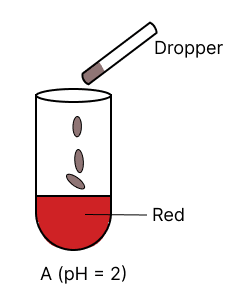
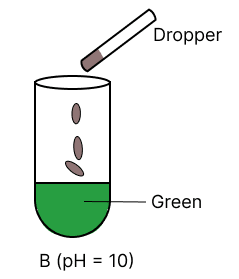
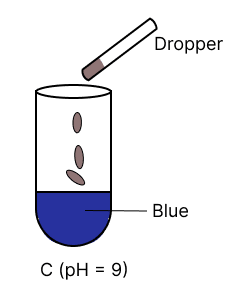
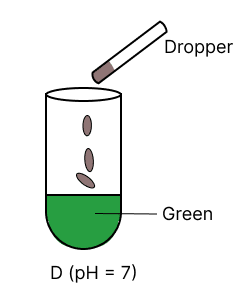
Assertion (A): Hydrogen is a neutral gas but it cannot be dried by concentrated sulphuric acid.
Reason (R): The gas reacts with this acid.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.