Chemistry
Assertion (A): Hydrogen is a neutral gas but it cannot be dried by concentrated sulphuric acid.
Reason (R): The gas reacts with this acid.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Sulphuric Acid
1 Like
Answer
Both A and R are true, and R is the correct explanation of A.
Reason — Hydrogen is neutral (neither acidic nor basic), and while concentrated H2SO4 is a drying agent, it cannot be used for drying hydrogen because concentrated H2SO4 is a strong oxidizing agent and may oxidize hydrogen, especially under certain conditions, which makes it unsafe and unsuitable. Hence, the assertion (A) is true.
Hydrogen gas reacts with concentrated H2SO4 because sulphuric acid is a strong oxidizing agent. Hence, the reason (R) is true and it explains why hydrogen cannot be dried with concentrated H2SO4.
Answered By
1 Like
Related Questions
The percentage of nitrogen present in urea (NH2)2CO is: [R.A.M. of N = 14, C= 12, O = 16, H = 1]
- 23.36
- 46.67
- 19.35
- 43.87
A few drops of universal indicator are added to colourless solution A, B, C and D with pH 2, 10, 9 and 7 respectively. Which of the following test tube is labelled with incorrect colour ?
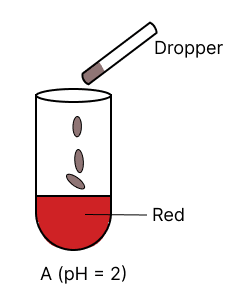
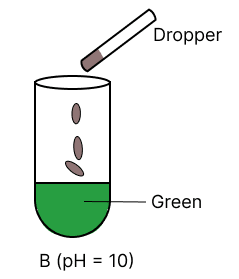
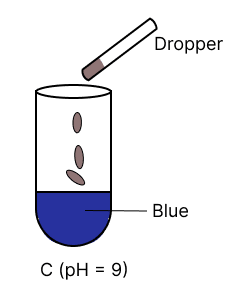
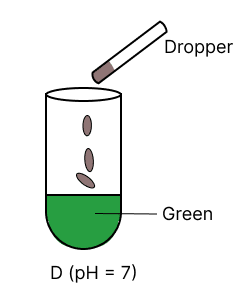
The organic compound prepared when calcium carbide reacts with water:
- Methane
- Ethane
- Acetylene
- Ethene
The IUPAC name of acetylene is:
- Propyne
- Ethene
- Propane
- Ethyne