Chemistry
In the following set up for burning ammonia in oxygen, ammonia tube is fitted higher and oxygen tube is kept lower because ?
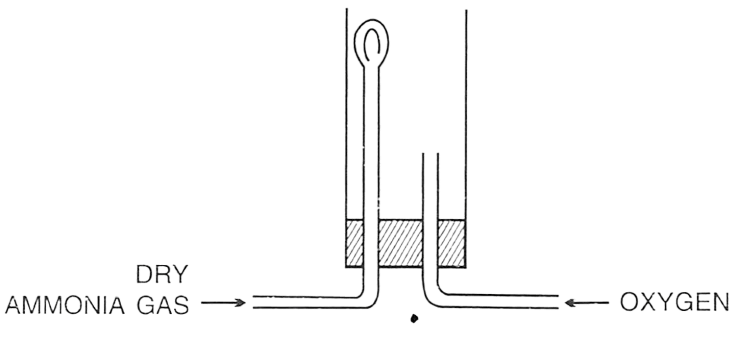
P — Oxygen is lighter than ammonia.
Q — Ammonia is lighter than oxygen.
R — Ammonia and oxygen mixture is explosive and by keeping them in this position, limited oxygen will react with ammonia.
Which of the following is true ?
- Only P
- Only Q
- Only R
- Both P and Q
Related Questions
When ammonia reacts with an excess of chlorine, the main product formed is:
- NH4Cl
- NCl3
- Cl2
- HCl
Ammonia is used in:
- Contact process
- Haber's process
- Ostwald process
- Bayer's process
Assertion (A): Ammonia does not conduct electricity in gaseous or liquid state.
Reason (R): Ions in ammonia gas or liquid ammonia are not free to move.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Assertion (A): Ammonia is dried by passing through a drying tower containing CaO.
Reason (R): Ammonia being basic in nature reacts with other drying agents.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.