Chemistry
When ammonia reacts with an excess of chlorine, the main product formed is:
- NH4Cl
- NCl3
- Cl2
- HCl
Ammonia
2 Likes
Answer
NCl3
Reason — When ammonia reacts with an excess of chlorine, products formed are hydrogen chloride and yellow coloured highly explosive liquid nitrogen trichloride.
NH3 + 3Cl2 ⟶ 3HCl + NCl3
Answered By
1 Like
Related Questions
The gas collected by downward displacement of air is
- HCl
- NH3
- CO2
- H2S
In the Haber's process, the ratio of reactants nitrogen and hydrogen is:
- 2 : 3
- 3 : 2
- 1 : 3
- 3 : 1
Ammonia is used in:
- Contact process
- Haber's process
- Ostwald process
- Bayer's process
In the following set up for burning ammonia in oxygen, ammonia tube is fitted higher and oxygen tube is kept lower because ?
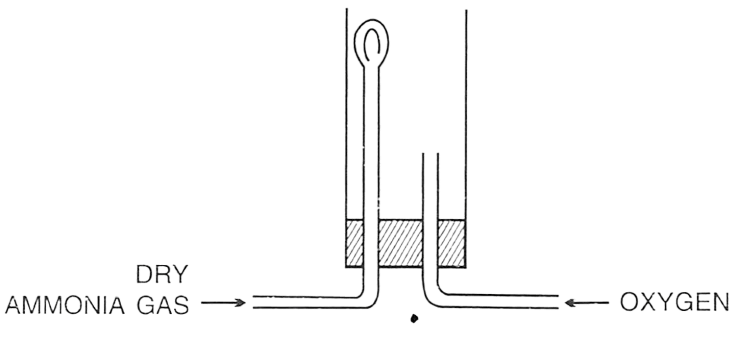
P — Oxygen is lighter than ammonia.
Q — Ammonia is lighter than oxygen.
R — Ammonia and oxygen mixture is explosive and by keeping them in this position, limited oxygen will react with ammonia.
Which of the following is true ?
- Only P
- Only Q
- Only R
- Both P and Q