Physics
During melting of ice at 0°C the :
- energy is released and temperature remains constant.
- energy is absorbed and temperature remains constant.
- energy is released and temperature decreases.
- energy is absorbed and temperature increases.
Related Questions
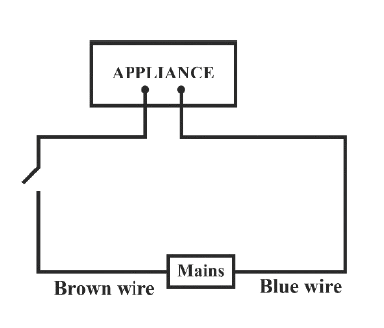
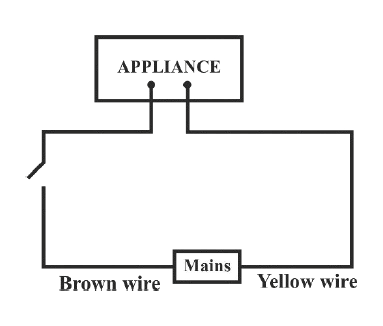
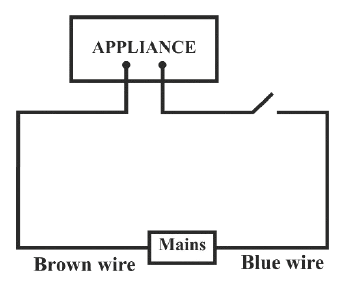
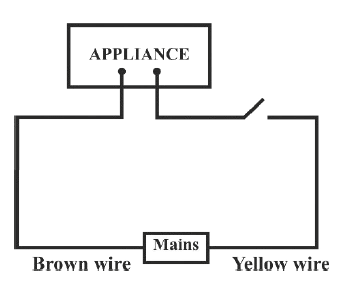
Identify the option that displays the correct wiring with correct colour code :
- 1.
- 2.
- 3.
- 4.
The potential difference between terminals of a cell in a closed electric circuit is:
- terminal voltage
- electro motive force
- voltage drop
- none of these
Linear magnification(m) produced by a concave lens is :
- m < 1
- m > 1
- m = 1
- m = 2
A radioactive element is placed in an evacuated chamber. Then the rate of radioactive decay will:
- Decrease
- Increase
- Remain unchanged
- Depend on the surrounding temperature