Physics
During melting of ice at 0°C the :
- energy is released and temperature remains constant.
- energy is absorbed and temperature remains constant.
- energy is released and temperature decreases.
- energy is absorbed and temperature increases.
Calorimetry
3 Likes
Answer
energy is absorbed and temperature remains constant.
Reason — When ice melts at 0°C, it absorbs heat energy from the surroundings which is used completely for phase change from solid (ice) to liquid (water) so this energy does not increase the temperature during the process.
Answered By
1 Like
Related Questions
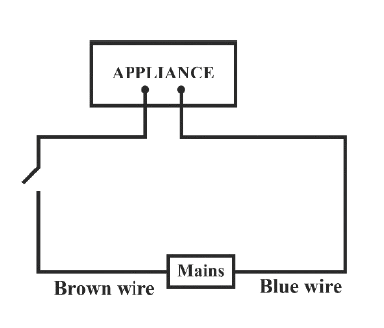
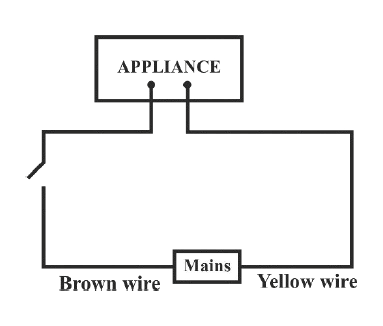
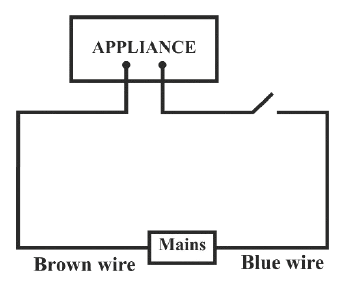
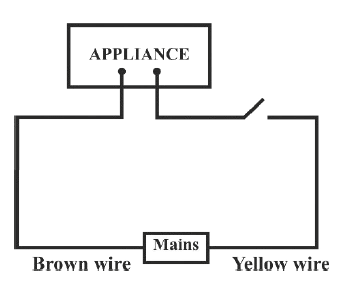
Identify the option that displays the correct wiring with correct colour code :
- 1.
- 2.
- 3.
- 4.
The potential difference between terminals of a cell in a closed electric circuit is:
- terminal voltage
- electro motive force
- voltage drop
- none of these
Linear magnification(m) produced by a concave lens is :
- m < 1
- m > 1
- m = 1
- m = 2
A radioactive element is placed in an evacuated chamber. Then the rate of radioactive decay will:
- Decrease
- Increase
- Remain unchanged
- Depend on the surrounding temperature