Chemistry
During the extraction of aluminium by Hall Heroult’s process, the carbon rods are replaced continuously. This is because:
- It minimises heat loss by radiation.
- It enhances the mobility of ions.
- The carbon anode is consumed.
- It lowers the fusion point.
Related Questions
10g of magnesium carbonate reacts completely with excess dilute hydrochloric acid. What volume of carbon dioxide is formed at room temperature and pressure? [Mg=24, C=12, O=16] The equation for the reaction is:
MgCO3 + 2HCl ⟶ MgCl2 + H2O + CO2
- 2.8 dm3
- 2.6 dm3
- 2.2 dm3
- 2.4 dm3
The diagram shown is a wrong attempt to electroplate a pan with copper:
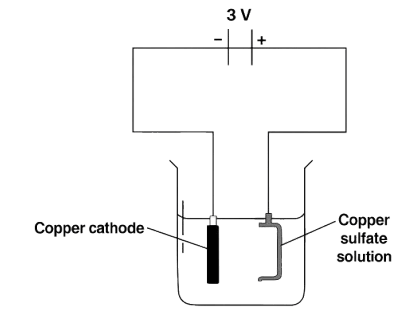
Which of the following could have been done to copper plate a pan?
- To change DC to AC.
- To change the electrolyte from copper sulphate to cobalt sulphate.
- Connect the pan to the negative electrode.
- To induce a higher current.
Which of the following observations correctly shows the action of indicator on sodium hydroxide solution?
Indicator methyl orange phenolphthalein 1. P orange to yellow remains colourless 2. Q orange to pink remains colourless 3. R orange to yellow colourless to pink 4. S remains orange remains pink When electrolysis of molten lead bromide is carried out, the products formed at the respective electrodes are:
At the positive electrons At the negative electrode 1. Bromine Lead 2. Bromine Hydrogen 3. Lead Bromine 4. Lead Oxygen