Chemistry
During the extraction of aluminium by Hall Heroult’s process, the carbon rods are replaced continuously. This is because:
- It minimises heat loss by radiation.
- It enhances the mobility of ions.
- The carbon anode is consumed.
- It lowers the fusion point.
Metallurgy
3 Likes
Answer
The carbon anode is consumed.
Reason — During the extraction of aluminium by Hall Heroult’s process, the carbon rod are replaced time to time, as it get oxidised by the oxygen evolved at the anode and get consumed.
Answered By
1 Like
Related Questions
10g of magnesium carbonate reacts completely with excess dilute hydrochloric acid. What volume of carbon dioxide is formed at room temperature and pressure? [Mg=24, C=12, O=16] The equation for the reaction is:
MgCO3 + 2HCl ⟶ MgCl2 + H2O + CO2
- 2.8 dm3
- 2.6 dm3
- 2.2 dm3
- 2.4 dm3
The diagram shown is a wrong attempt to electroplate a pan with copper:
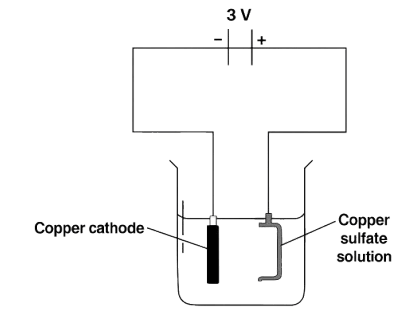
Which of the following could have been done to copper plate a pan?
- To change DC to AC.
- To change the electrolyte from copper sulphate to cobalt sulphate.
- Connect the pan to the negative electrode.
- To induce a higher current.
Which of the following observations correctly shows the action of indicator on sodium hydroxide solution?
Indicator methyl orange phenolphthalein 1. P orange to yellow remains colourless 2. Q orange to pink remains colourless 3. R orange to yellow colourless to pink 4. S remains orange remains pink When electrolysis of molten lead bromide is carried out, the products formed at the respective electrodes are:
At the positive electrons At the negative electrode 1. Bromine Lead 2. Bromine Hydrogen 3. Lead Bromine 4. Lead Oxygen