Chemistry
Answer
- 2.
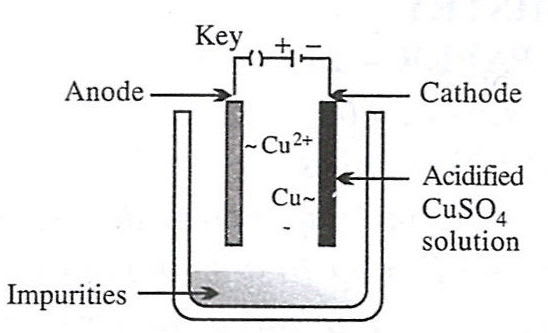
Reason — Electrolytic refining is a process by which metals containing impurities are purified electrolytically to give a pure metal. Option 2 shows the set-up used for electrolytic refining of copper:
- Anode – impure copper block (loses electrons and goes into solution).
Cu - 2e- ⟶ Cu2+ - Cathode – thin sheet of pure copper (gains mass as metal is deposited).
Cu2+ + 2e- ⟶ Cu - Electrolyte – acidified aqueous CuSO₄ solution.
As current passes, pure copper is transferred from the impure anode to the cathode, while insoluble impurities collect below the anode as "anode mud".
Related Questions
In the given equation, identify the role played by concentrated sulphuric acid.
S + 2H2SO4 ⟶ 3SO2 + 2H2O
- Non-volatile acid
- Oxidising agent
- Dehydrating agent
- None of the above
Nitrogen gas can be obtained by heating
- Ammonium nitrate
- Ammonium nitrite
- Magnesium nitride
- Ammonium chloride
Assertion (A): The RMM of a gas is 64, then its vapour density is 32.
Reason (R): RMM is twice the vapour density.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
The metals zinc and tin are present in the alloy
- Solder
- Brass
- Bronze
- Duralumin