Chemistry
In the given equation, identify the role played by concentrated sulphuric acid.
S + 2H2SO4 ⟶ 3SO2 + 2H2O
- Non-volatile acid
- Oxidising agent
- Dehydrating agent
- None of the above
Sulphuric Acid
1 Like
Answer
Oxidising agent
Reason — Concentrated sulphuric acid acts as a oxidising agent, it is due to the fact that on thermal decomposition it yields nascent oxygen [O].
H2SO4 ⟶ H2O + SO2 + [O]
Nascent oxygen oxidises non-metals, metals and inorganic compounds.
C + 2H2SO4 ⟶ CO2 + 2H2O + 2SO2 ↑
Answered By
3 Likes
Related Questions
Which of these will act as a non-electrolyte?
- Liquid carbon tetrachloride
- Acetic acid
- Sodium hydroxide aqueous solution acid
- Potassium chloride aqueous solution
Assertion (A): Carbon monoxide will not produce an acid when made to react with water?
Reason (R): Carbon monoxide is a neutral oxide.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Nitrogen gas can be obtained by heating
- Ammonium nitrate
- Ammonium nitrite
- Magnesium nitride
- Ammonium chloride
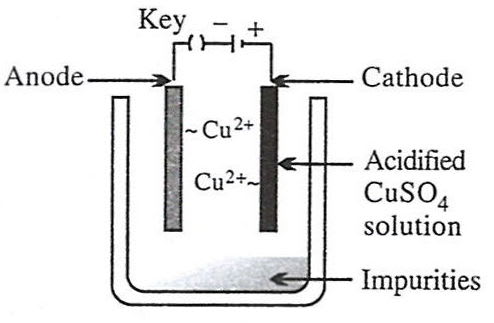
Which of the following figures correctly describes electrorefining ?
- 1.
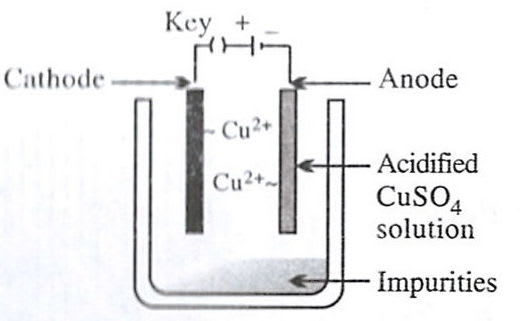
- 2.
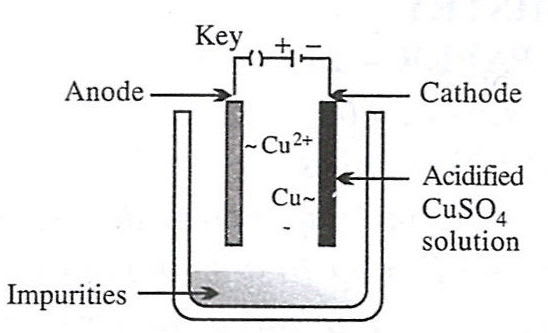
- 3.
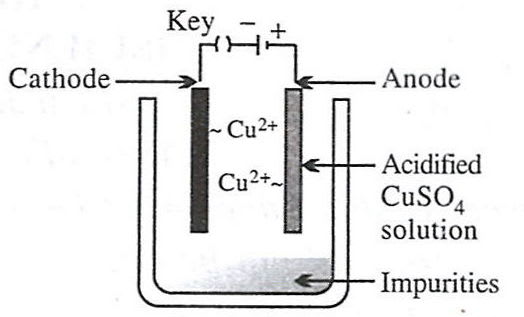
- 4.