Chemistry
Which of the following figures correctly describes electrorefining ?
- 1.
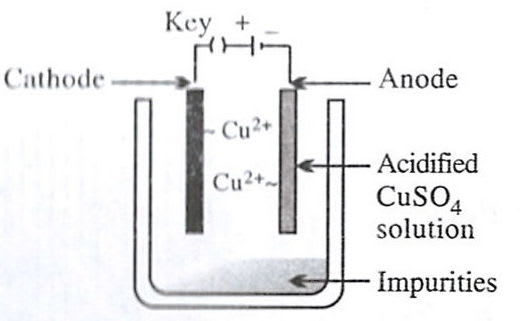
- 2.
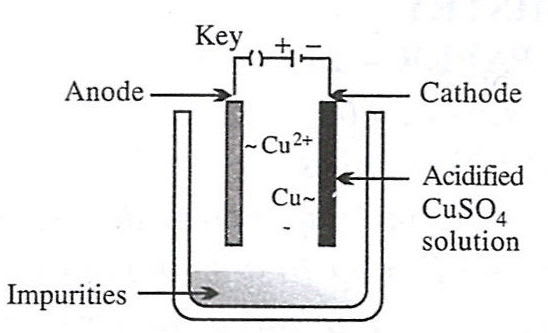
- 3.
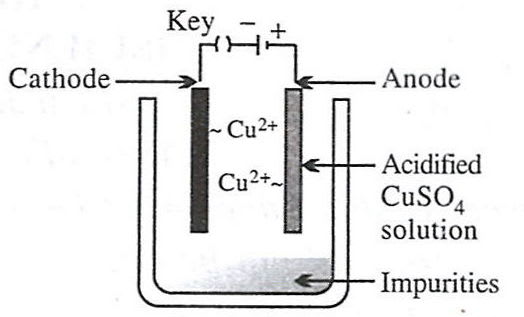
- 4.
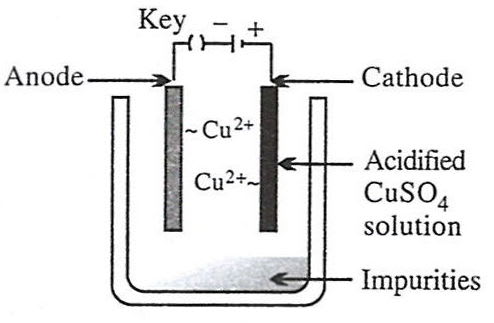
Metallurgy
2 Likes
Answer
- 2.

Reason — Electrolytic refining is a process by which metals containing impurities are purified electrolytically to give a pure metal. Option 2 shows the set-up used for electrolytic refining of copper:
- Anode – impure copper block (loses electrons and goes into solution).
Cu - 2e- ⟶ Cu2+ - Cathode – thin sheet of pure copper (gains mass as metal is deposited).
Cu2+ + 2e- ⟶ Cu - Electrolyte – acidified aqueous CuSO₄ solution.
As current passes, pure copper is transferred from the impure anode to the cathode, while insoluble impurities collect below the anode as "anode mud".
Answered By
3 Likes
Related Questions
In the given equation, identify the role played by concentrated sulphuric acid.
S + 2H2SO4 ⟶ 3SO2 + 2H2O
- Non-volatile acid
- Oxidising agent
- Dehydrating agent
- None of the above
Nitrogen gas can be obtained by heating
- Ammonium nitrate
- Ammonium nitrite
- Magnesium nitride
- Ammonium chloride
Assertion (A): The RMM of a gas is 64, then its vapour density is 32.
Reason (R): RMM is twice the vapour density.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
The metals zinc and tin are present in the alloy
- Solder
- Brass
- Bronze
- Duralumin