Chemistry
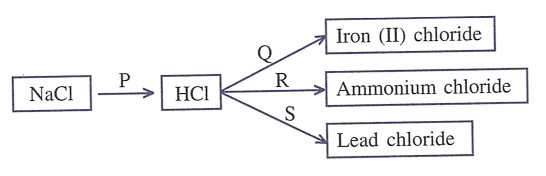
Refer to the diagram given below and write balanced equations with conditions, if any, for the following conversions P to S.
Related Questions
In the laboratory preparation of hydrochloric acid, hydrogen chloride gas is dissolved in water.
(i) Draw a diagram to show the arrangement used for the absorption of HCl gas in water.
(ii) State why such an arrangement is necessary? Give two reasons for the same.
(iii) Write balanced chemical equations for the laboratory preparation of HCl gas when the reactants are :
(A) below 200°C
(B) above 200°C
Study the figure given below and answer the questions that follow :

(i) Identify the gas Y.
(ii) What property of gas Y does this experiment demonstrate?
(iii) Name another gas which has the same property and can be demonstrated through this experiment.
State your observations:
(a) HCl gas is passed through lead nitrate solution and the mixture is heated.
(b) Hydrochloric acid is added to silver nitrate solution.
(c) Ammonium hydroxide solution is added to the resultant product of part (b).
Distinguish by using HCl.
(a) Lead nitrate solution and silver nitrate solution.
(b) Potassium sulphite and potassium sulphide.