Chemistry
Distinguish by using HCl.
(a) Lead nitrate solution and silver nitrate solution.
(b) Potassium sulphite and potassium sulphide.
Hydrogen Chloride
12 Likes
Answer
(a) On reaction with HCl, lead nitrate solution gives white precipitate which can be dissolved in hot water.
Pb(NO3)2 + 2HCl ⟶ PbCl2 ↓ + 2HNO3
Whereas, silver nitrate solution gives thick curdy white precipitate which is insoluble in hot water.
AgNO3 + HCl ⟶ AgCl ↓ + HNO3
(b) On reaction with HCl, potassium sulphite gives SO2 gas with pungent odour
K2SO3 + 2HCl ⟶ 2KCl + H2O + SO2 ↑
However, potassium sulphide gives H2S gas with rotten eggs odour.
K2S + 2HCl ⟶ 2KCl + H2S ↑
Answered By
7 Likes
Related Questions
Refer to the diagram given below and write balanced equations with conditions, if any, for the following conversions P to S.
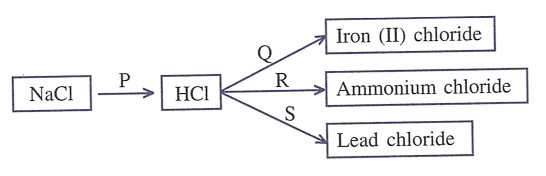
State your observations:
(a) HCl gas is passed through lead nitrate solution and the mixture is heated.
(b) Hydrochloric acid is added to silver nitrate solution.
(c) Ammonium hydroxide solution is added to the resultant product of part (b).
The following questions pertain to the laboratory preparation of hydrogen chloride gas:
(i) Write the equation for it's preparation mentioning the condition required.
(ii) Name the drying agent used in the above preparation and give a reason for the choice.
(iii) State a safety precaution taken during the preparation of hydrochloric acid.
Identify the gas evolved and give the chemical test when dilute hydrochloric acid reacts with :
(i) Sodium sulphite.
(ii) Iron [II] sulphide.