Chemistry
Assertion (A): The atomic mass of sodium is 23 amu.
Reason (R): An atom of sodium is 23 times heavier than an atom of carbon with mass 12 amu.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is true but R is false.
Explanation — The atomic mass of sodium is 23 amu. Hence, the assertion (A) is true.
An atom of sodium is 23 times heavier than one-twelfth the mass of a carbon-12 atom, not 23 times heavier than an atom of carbon with mass 12 amu. Hence, reason (R) is false.
Related Questions
Copper shows variable valency and forms two different compounds with oxygen — Cu2O and CuO.
P — A is cuprous oxide, B is cupric oxide.
Q — A is cupric oxide, B is cuprous oxide.
R — A is copper (I) oxide, B is copper (II) oxide.
Only P
Only Q
Only R
Both P and R
The figure given below shows the molecule of an element, where 'a' denotes the atom with atomic mass 32.
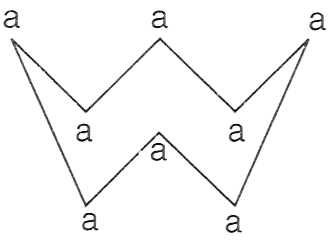
P — Element is tetratomic with molecular mass 128.
Q — Element is octatomic with molecular mass 256.
R — Element is crown-shaped with molecular mass 256.
Only P
Only Q
Only R
Both P and R
Assertion (A): An atom is the smallest part of matter which can take part in a chemical reaction.
Reason (R): Atoms of every element can exist independently.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Assertion (A): All equations need to be balanced.
Reason (R): An unbalanced equation would imply that atoms have been created or destroyed.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.