Chemistry
Copper shows variable valency and forms two different compounds with oxygen — Cu2O and CuO.
P — A is cuprous oxide, B is cupric oxide.
Q — A is cupric oxide, B is cuprous oxide.
R — A is copper (I) oxide, B is copper (II) oxide.
Only P
Only Q
Only R
Both P and R
Valency
3 Likes
Answer
Both P and R
Reason — Certain elements exhibit more than one valency and they show variable valency.
For example, copper shows valency of 1 and 2 and forms compounds like, cuprous [copper(I)] oxide and cupric [copper(II)] oxide, respectively.
Hence, both P and R are true.
Answered By
1 Like
Related Questions
One "μ" stands for :
- One atom of any element
- 1/12th of hydrogen atom
- An atom of carbon (C-12)
- 1/12th the mass of carbon atom (C-12).
In the compound magnesium nitride (Mg3N2):
P — Mg is trivalent, N is divalent.
Q — Mg is divalent. N is trivalent.
R — Mg is divalent, N is divalent.
Which of the statements given above is/are true?
Only P
Only Q
Only R
Both P and Q
The figure given below shows the molecule of an element, where 'a' denotes the atom with atomic mass 32.
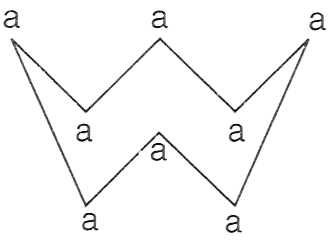
P — Element is tetratomic with molecular mass 128.
Q — Element is octatomic with molecular mass 256.
R — Element is crown-shaped with molecular mass 256.
Only P
Only Q
Only R
Both P and R
Assertion (A): The atomic mass of sodium is 23 amu.
Reason (R): An atom of sodium is 23 times heavier than an atom of carbon with mass 12 amu.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.