Chemistry
Assertion (A): An atom is the smallest part of matter which can take part in a chemical reaction.
Reason (R): Atoms of every element can exist independently.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Chemical Formula
1 Like
Answer
A is true but R is false.
Explanation — An atom is the smallest particle of an element and can take part in a chemical reaction. Hence, the assertion (A) is true.
Atoms may or may not exist independently.
For example:
Noble gas atoms like He, Ne can exist freely.
But atoms like H, O, and N do not exist alone under normal conditions; they exist as diatomic molecules (H2, O2, N2). Hence, reason (R) is false.
Answered By
2 Likes
Related Questions
The figure given below shows the molecule of an element, where 'a' denotes the atom with atomic mass 32.
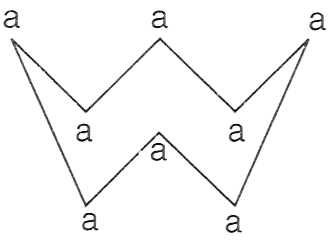
P — Element is tetratomic with molecular mass 128.
Q — Element is octatomic with molecular mass 256.
R — Element is crown-shaped with molecular mass 256.
Only P
Only Q
Only R
Both P and R
Assertion (A): The atomic mass of sodium is 23 amu.
Reason (R): An atom of sodium is 23 times heavier than an atom of carbon with mass 12 amu.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Assertion (A): All equations need to be balanced.
Reason (R): An unbalanced equation would imply that atoms have been created or destroyed.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Assertion (A): PCl3, is known as phosphorus trichloride while AlCl3, is aluminium chloride and not aluminium trichloride.
Reason (R) : Phosphorus shows variable valency. Aluminium does not show variable valency
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.