Chemistry
Which of the following arrangements is INCORRECT as per the property stated against it?
- Li > Be > N > O (Metallic character)
- CI > F > Br > I (Electron gain enthalpy)
- O2- > F - > Mg2+ > Na+(Ionic radii)
- I > Br > CI > F (Number of shells)
Answer
O2- > F - > Mg2+ > Na+(Ionic radii)
Reason —
| Periodic property | Arrangement | Explanation |
|---|---|---|
| Metallic character | Li > Be > N > O | Metallic character decreases across a period from left to right |
| Electron gain enthalpy | CI > F > Br > I | Electron gain enthalpy generally becomes less negative down a group |
| Ionic radii | O2- > F - > Mg2+ > Na+ | Ionic radius of isoelectronic species (same number of electrons), decreases with increasing nuclear charge |
| Number of shells | I > Br > CI > F | Number of shells increases down the group. I has the most shells |
In case of ionic radius, O2- > F - > Mg2+ > Na+ has 10 electrons each, Atomic numbers(Z) of O = 8, F = 9, Na = 11, Mg = 12, Higher the Z, greater is the attraction and smaller is the radius.
The correct order would be, O2- > F - > Na+ > Mg2+.
Related Questions
The following are the structural diagrams of certain hydrocarbons:
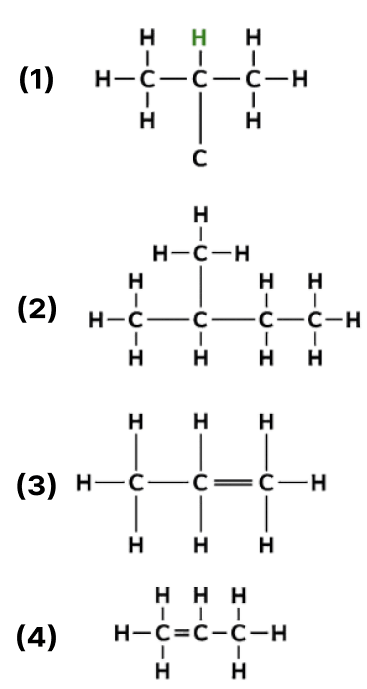
Which two structures are related to each other?
- A and B
- B and C
- C and D
- A and C
The electronic configuration of X is 2,8,6. It gains 'Y’ electrons into its valence shell to attain the nearest noble gas electronic configuration and gets converted to an ion Z. X, Y, and Z, respectively, are:
- Sodium, one, electropositive
- Beryllium, two, electronegative
- Oxygen, six, electronegative
- Sulphur, two, electronegative
Baking soda (NaHCO3), when added to vinegar, evolves a gas. Which of these statements is true about the evolution of gas?
I. It turns limewater milky.
II. It extinguishes the burning splinter.
III. It acts as a non-metallic oxide
IV. It has a pungent odour.- I and IV
- I and II
- I, II and III
- III and IV
The statements below show the results when three metal strips, P, Q, and R, are placed in blue copper sulphate solution.
P — Solution turns green. Q — Solution becomes colourless. R — Solution remains blue.
Which of the following metals could be P, Q, and R?
- P-Al, Q-Zn, R- Fe
- P-Zn, Q-Fe, R- Ag
- P-Fe, Q-Zn, R-Ag
- P- Zn, Q-AI, R– Fe