Chemistry
Baking soda (NaHCO3), when added to vinegar, evolves a gas. Which of these statements is true about the evolution of gas?
I. It turns limewater milky.
II. It extinguishes the burning splinter.
III. It acts as a non-metallic oxide
IV. It has a pungent odour.
- I and IV
- I and II
- I, II and III
- III and IV
Organic Chemistry
2 Likes
Answer
I, II and III
Reason — When baking soda (NaHCO3), added to vinegar (acetic acid), CO2 is evolved. CO2 turns limewater milky, extinguishes the burning splinter and acts as a non-metallic oxide.
CH3COOH + NaHCO3 ⟶ CH3COONa + H2O + CO2 ↑
CO2 has no pungent odour.
Answered By
1 Like
Related Questions
The electronic configuration of X is 2,8,6. It gains 'Y’ electrons into its valence shell to attain the nearest noble gas electronic configuration and gets converted to an ion Z. X, Y, and Z, respectively, are:
- Sodium, one, electropositive
- Beryllium, two, electronegative
- Oxygen, six, electronegative
- Sulphur, two, electronegative
Which of the following arrangements is INCORRECT as per the property stated against it?
- Li > Be > N > O (Metallic character)
- CI > F > Br > I (Electron gain enthalpy)
- O2- > F - > Mg2+ > Na+(Ionic radii)
- I > Br > CI > F (Number of shells)
The statements below show the results when three metal strips, P, Q, and R, are placed in blue copper sulphate solution.
P — Solution turns green. Q — Solution becomes colourless. R — Solution remains blue.
Which of the following metals could be P, Q, and R?
- P-Al, Q-Zn, R- Fe
- P-Zn, Q-Fe, R- Ag
- P-Fe, Q-Zn, R-Ag
- P- Zn, Q-AI, R– Fe
Study the below diagram and choose the correct option related to the content given below:
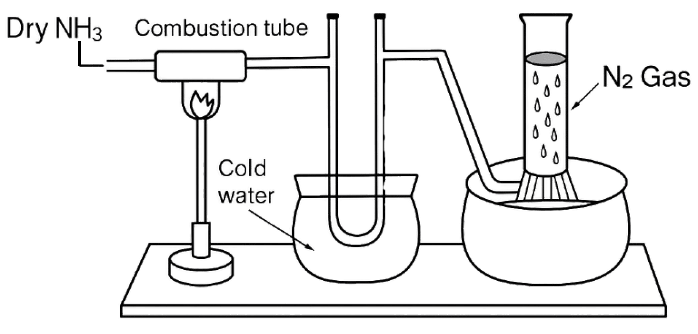
Compound X reacts with ammonia in the combustion tube, which leaves a residue Y. Identify X and Y, as well as the property Z of ammonia demonstrated in this particular reaction.
- X= CuO, Y=black, Z = reducing property.
- X=PbO, Y = yellow, Z=oxidising property.
- X=CuO, Y =yellow, Z =oxidising property.
- X=PbO, Y=black, Z=reducing property.