When 2 mL of sodium hydroxide solution is added to few pieces of granulated zinc in a test tube and then warmed, the reaction that occurs can be written in the form of a balanced chemical equation as :
options
- NaOH + Zn → NaZnO2 + H2O
- 2NaOH + Zn → Na2ZnO2 + H2
- 2NaOH + Zn → NaZnO2 + H2
- 2NaOH + Zn → Na2ZnO2 + H2O
Answer
2NaOH + Zn → Na2ZnO2 + H2
Reason — When a few pieces of granulated zinc are added to about 2 mL of sodium hydroxide solution in a test tube and the mixture is warmed, a chemical reaction takes place in which bubbles of hydrogen gas are evolved.
This happens because zinc is an amphoteric metal, meaning it can react with both acids and bases so in this case, zinc reacts with the strong base sodium hydroxide to form a soluble compound called sodium zincate and hydrogen gas.
The reaction can be represented by the balanced chemical equation:
2NaOH + Zn → Na2ZnO2 + H2
Select from the following a decomposition reaction in which source of energy for decomposition is light:
options
- 2FeSO4 → Fe2O3 + SO2 + SO3
- 2H2O → 2H2 + O2
- 2AgBr → 2Ag + Br2
- CaCO3 → CaO + CO2
Answer
2AgBr → 2Ag + Br2
Reason —
A decomposition reaction whose energy source is light is called a photochemical decomposition. Silver bromide (AgBr) is well known for this because when AgBr is exposed to light, photons supply the energy needed to break chemical bonds, producing metallic silver and bromine according to
A metal and a non-metal that exists in liquid state at the room temperature are respectively:
options
- Bromine and Mercury
- Mercury and Iodine
- Mercury and Bromine
- Iodine and Mercury
Answer
Mercury and Bromine
Reason — At room temperature, most metals and non-metals are solids, but there are a few exceptions :
- Mercury (Hg) is the only metal that exists as a liquid at room temperature (around 25 °C) and it remains in liquid form because of its weak metallic bonding and low melting point (−38.83 °C).
- Bromine (Br2) is the only non-metal that is liquid at room temperature. It has a deep reddish-brown color and easily evaporates to form brown fumes.
Thus, the metal and non-metal that exist in the liquid state at room temperature are Mercury and Bromine, respectively.
Carbon compounds :
(a) are good conductors of electricity.
(b) are bad conductors of electricity.
(c) have strong forces of attraction between their molecules.
(d) have weak forces of attraction between their molecules.
The correct statements are:
options
- (a) and (b)
- (b) and (c)
- (b) and (d)
- (a) and (c)
Answer
(b) and (d)
Reason — Most carbon compounds such as methane, ethanol, glucose and other organic substances are covalent in nature.
Because of covalent bonding, these compounds do not have free electrons or ions available to carry electric current. Hence, they are bad conductors of electricity.
Also, in covalent compounds, the forces of attraction between molecules are weak, which is why many carbon compounds exist as liquids or gases and have relatively low melting and boiling points compared to ionic compounds.
Therefore, the correct statements are:
(b) Carbon compounds are bad conductors of electricity.
(d) Carbon compounds have weak forces of attraction between their molecules.
Consider the following compounds :
FeSO4; CuSO4; CaSO4; Na2CO3
The compound having maximum number of water of crystallisation in its crystalline form in one molecule is :
options
- FeSO4
- CuSO4
- CaSO4
- Na2CO3
Answer
Na2CO3
Reason — Among the given salts their common crystalline hydrates are :
- Ferrous sulphate heptahydrate (FeSO4·7H2O),
- Copper sulphate pentahydrate (CuSO4·5H2O),
- Gypsum/Calcium sulphate dihydrate (CaSO4·2H2O), and
- Washing soda/Sodium carbonate decahydrate (Na2CO3·10H2O).
Since 10 > 7 > 5 > 2, sodium carbonate (Na2CO3·10H2O) contains the largest number of water molecules per formula unit.
Oxides of aluminium and zinc are:
options
- Acidic
- Basic
- Amphoteric
- Neutral
Answer
Amphoteric
Reason —
- When oxides of aluminium and zinc react with acids like HCl, they behave as bases and form salts such as aluminium chloride or zinc chloride.
Al2O3 + 6HCl → 2AlCl3 + 3H2O
ZnO + 2HCl → ZnCl2 + H2O
- When oxides of aluminium and zinc react with alkalis like NaOH, they behave as acids and form complex salts such as sodium aluminate or sodium zincate.
Al2O3 + 2NaOH → 2NaAlO2 + H2O
ZnO + 2NaOH → Na2ZnO2 + H2O
Because oxides of aluminium and zinc react with both acids and bases, these oxides are classified as amphoteric oxides.
Hence, Aluminium oxide (Al2O3) and zinc oxide (ZnO) both show amphoteric behaviour.
The reaction given below is a redox reaction because in this case :
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
options
- MnO2 is oxidised and HCl is reduced.
- HCl is oxidised.
- MnO2 is reduced.
- MnO2 is reduced and HCl is oxidised.
Answer
MnO2 is reduced and HCl is oxidised
Reason — When manganese dioxide (MnO2) reacts with hydrochloric acid (HCl), chlorine gas (Cl2) is released. In this reaction,
- HCl gives chlorine gas, which means HCl is oxidised and
- MnO2 changes to MnCl2, showing that MnO2 is reduced.
Since one substance (HCl) is oxidised and another (MnO2) is reduced, this reaction is called a redox reaction.
Consider the following statements :
(a) The sex of a child is determined by what it inherits from the mother.
(b) The sex of a child is determined by what it inherits from the father.
(c) The probability of having a male child is more than that of a female child.
(d) The sex of a child is determined at the time of fertilisation when male and female gametes fuse to form a zygote.
The correct statements are:
options
- (a) and (c)
- (b) and (d)
- (c) and (d)
- (a), (c) and (d)
Answer
(b) and (d)
Reason —
- Statement (a) is incorrect.
Corrected statement : The mother’s egg always carries an X chromosome, so she cannot determine the sex of the child.
Statement (b) is correct.
Statement (c) is incorrect.
Corrected statement : The probability of having a boy or girl is equal (50–50 chance) because half of the sperms carry X and half carry Y.
- Statement (d) is correct.
Hence, correct statements are (b) and (d).
Chromosomes :
(a) carry hereditary information from parents to the next generation.
(b) are thread like structures located inside the nucleus of an animal cell.
(c) always exist in pairs in human reproductive cells.
(d) are involved in the process of cell division.
The correct statements are :
options
- (a) and (b)
- (c) and (d)
- (a), (b) and (d)
- (a) and (d)
Answer
(a), (b) and (d)
Reason — Properties of chromosomes :
- Chromosomes are thread-like structures found inside the nucleus of a cell.
- They are made up of DNA and proteins and play a vital role in carrying hereditary information from parents to offspring.
- This genetic information is passed through genes present on the chromosomes, which determine various traits in living organisms.
- Chromosomes are also actively involved during cell division, where they ensure that the genetic material is equally distributed between the daughter cells.
- In human reproductive cells such as sperms and eggs, chromosomes do not exist in pairs; they are present as a single set (haploid).
Therefore, the correct statements about chromosomes are that they carry hereditary information, are thread-like structures in the nucleus, and are involved in cell division.
In a nerve cell, the site where the electrical impulse is converted into a chemical signal is known as:
options
- Axon
- Dendrites
- Neuromuscular junction
- Cell body
Answer
Neuromuscular junction
Reason — At the neuromuscular junction (a type of synapse between a motor neuron and a muscle fibre), the electrical impulse travelling along the axon reaches the axon terminal. Here it stimulates the release of neurotransmitters, which are chemical messengers. Thus, the electrical impulse is converted into a chemical signal at the neuromuscular junction.
A stomata closes when :
(a) it needs carbon dioxide for photosynthesis.
(b) it does not need carbon dioxide for photosynthesis.
(c) water flows out of the guard cells.
(d) water flows into the guard cells.
The correct reason(s) in this process is/are:
options
- (a) only
- (a) and (c)
- (b) and (c)
- (b) and (d)
Answer
(b) and (c)
Reason — Stomata are tiny pores on the surface of leaves that control the exchange of gases and water.
When a plant does not need carbon dioxide for photosynthesis or wants to prevent water loss, the guard cells lose water and become flaccid, causing the stomata to close.
So, stomata close when:
- it does not need carbon dioxide for photosynthesis, and
- water flows out of the guard cells.
Hence, correct reasons are (b) and (c).
At what distance from a convex lens should an object be placed to get an image of the same size as that of the object on a screen?
options
- Beyond twice the focal length of the lens.
- At the principal focus of the lens.
- At twice the focal length of the lens.
- Between the optical centre of the lens and its principal focus.
Answer
At twice the focal length of the lens.
Reason — When an object is placed at a distance equal to twice the focal length (2f) from a convex lens, the image formed is real, inverted and of the same size as the object.
The lens system of human eye forms an image on a light sensitive screen, which is called as :
options
- Cornea
- Ciliary muscles
- Optic nerves
- Retina
Answer
Retina
Reason — In the human eye, the lens system focuses light rays coming from an object onto a light-sensitive screen located at the back of the eye which is called the retina.
The retina contains special light-sensitive cells known as rods and cones, which detect the light and convert it into electrical signals which are then sent to the brain through the optic nerve, allowing us to see the image.
The pattern of the magnetic field produced inside a current carrying solenoid is:
options

Answer

Reason — Option a is correct answer because inside a current-carrying solenoid, the magnetic field lines are straight, parallel and closely spaced, showing that the field is strong and uniform throughout the solenoid.
Outside the solenoid, the field resembles that of a bar magnet, with lines emerging from one end (north pole) and entering the other (south pole).
Among the given diagrams, the one showing parallel and equally spaced magnetic field lines inside the solenoid corresponds to this pattern.
Identify the food chain in which the organisms of the second trophic level are missing:
options
- Grass, goat, lion
- Zooplankton, Phytoplankton, small fish, large fish
- Tiger, grass, snake, frog
- Grasshopper, grass, snake, frog, eagle
Answer
Tiger, grass, snake, frog
Reason — A food chain’s first trophic level is made up of producers (plants) and the second trophic level contains primary consumers (herbivores) that feed on those producers.
In the food chain — Tiger, grass, snake, frog — grass (a producer) is present but there is no herbivore shown that eats the grass before higher predators appear. The chain therefore lacks the primary-consumer (second trophic) level.
In which of the following organisms, multiple fission is a means of asexual reproduction?
options
- Yeast
- Leishmania
- Paramoecium
- Plasmodium
Answer
Plasmodium
Reason — Multiple fission is a type of asexual reproduction in which the parent cell divides into many daughter cells at the same time which occurs in Plasmodium, the parasite that causes malaria.
Inside its host’s liver or red blood cells, Plasmodium’s nucleus divides several times before the cell splits into many smaller cells, each forming a new organism.
Assertion (A) : Hydrogen gas is not evolved when zinc reacts with nitric acid.
Reason (R) : Nitric acid oxidises the hydrogen gas produced to water and itself gets reduced.
options
- (A) and (R) are true and (R) is the reason for (A).
- (A) and (R) are true and (R) is not the reason for (A).
- (A) is true, but (R) is false.
- (A) is false, but (R) is true.
Answer
(A) and (R) are true and (R) is the reason for (A)
Reason —
Assertion (A) is true because normally, when zinc reacts with acids like hydrochloric acid or sulphuric acid, hydrogen gas is released but since nitric acid is a strong oxidising agent so when zinc reacts with nitric acid, instead of releasing hydrogen gas, the hydrogen formed is immediately oxidised by nitric acid to form water.
Reason (R) is true because during the reaction, nitric acid acts as an oxidising agent so it oxidises the hydrogen (which would have formed as gas) into water and nitric acid itself gets reduced to nitrogen oxides such as NO or NO2.
Hence, (A) and (R) are true and (R) is the reason for (A)
Assertion (A) : Accumulation of harmful chemicals is maximum in the organisms at the highest trophic level of a food chain.
Reason (R) : Harmful chemicals are sprayed on the crops to protect them from diseases and pests.
options
- (A) and (R) are true and (R) is the reason for (A).
- (A) and (R) are true and (R) is not the reason for (A).
- (A) is true, but (R) is false.
- (A) is false, but (R) is true.
Answer
(A) and (R) are true and (R) is not the reason for (A).
Reason —
Assertion (A) is true because this happens due to biological magnification, where harmful chemicals such as pesticides or insecticides enter the food chain and their concentration increases at each successive trophic level. Thus, the organisms at the highest trophic level (like hawks or humans) accumulate the maximum amount of these toxic substances in their bodies.
Reason (R) is also true because pesticides and insecticides are sprayed on crops to kill pests and prevent plant diseases. These chemicals, however, get absorbed by plants and enter the food chain when animals or humans consume these plants.
Both Assertion (A) and Reason (R) are true, but Reason (R) only gives the cause of entry of harmful chemicals into the food chain; it does not directly explain why their accumulation is maximum at the highest trophic level.
Hence, (A) and (R) are true and (R) is not the reason for (A).
Assertion (A) : The rate of breathing in aquatic organisms is much faster than in terrestrial organisms.
Reason (R) : The amount of oxygen dissolved in water is very high as compared to the amount of oxygen in air.
options
- (A) and (R) are true and (R) is the reason for (A).
- (A) and (R) are true and (R) is not the reason for (A).
- (A) is true, but (R) is false.
- (A) is false, but (R) is true.
Answer
(A) is true, but (R) is false.
Reason —
Assertion (A) is true because aquatic organisms like fish have to take in water continuously through their gills to obtain the oxygen dissolved in it. Since the amount of oxygen available in water is very limited, they must breathe faster to meet their energy needs.
Reason (R) is false because the amount of oxygen dissolved in water is much lower than the amount of oxygen present in air. That’s why aquatic organisms need to breathe faster — to extract enough oxygen from the limited supply in water.
Hence, (A) is true, but (R) is false.
Assertion (A) : The rainbow is a natural spectrum of sunlight in the sky.
Reason (R) : Rainbow is formed in the sky when the sun is overhead and water droplets are also present in air.
options
- (A) and (R) are true and (R) is the reason for (A).
- (A) and (R) are true and (R) is not the reason for (A).
- (A) is true, but (R) is false.
- (A) is false, but (R) is true.
Answer
(A) is true, but (R) is false.
Reason —
Assertion (A) is true because a rainbow is indeed a natural spectrum that appears when sunlight is dispersed by water droplets in the atmosphere and the white light of the sun splits into its seven constituent colours — violet, indigo, blue, green, yellow, orange, and red.
Reason (R) is false because a rainbow is not formed when the sun is overhead but it is formed when the sun is low in the sky, usually in the morning or evening and water droplets from rain or mist are present opposite the sun.
Hence, (A) is true, but (R) is false.
Name the type of chemical reaction in which calcium oxide reacts with water. Justify your answer by giving balanced chemical equation for the chemical reaction.
Answer
Combination reaction
CaO + H2O → Ca(OH)2
Reason — One molecule of calcium oxide combines with one molecule of water to give one molecule of calcium hydroxide, so atoms of Ca, O and H are balanced on both sides. This reaction is also exothermic (called the slaking of lime), because heat is released when calcium oxide combines with water.
State one role of each of the following in human digestive system:
(a) Hydrochloric acid
(b) Villi
(c) Anal Sphincter
(d) Lipase
Answer
(a) Hydrochloric acid : Makes the food acidic in the stomach to provide the medium required for pepsin to act on proteins.
(b) Villi : Increase the surface area of the small intestine for absorption of digested food into the blood.
(c) Anal sphincter : Controls the opening and closing of the anus, thereby regulating the removal of undigested food (faeces) from the body.
(d) Lipase : Digests fats into fatty acids and glycerol.
How is the movement of leaves of a sensitive plant different from the downward movement of the roots?
Answer
The movement of leaves in a sensitive plant (like Mimosa pudica) occurs in response to touch or contact. It is a nastic movement, which means it does not depend on the direction of the stimulus and this movement happens due to changes in water content in the cells of the leaf base, not due to growth.
The downward movement of roots, on the other hand, is a tropic movement, specifically geotropism or gravitropism, which means it occurs in response to the direction of gravity and involves growth of the root cells.
There is a hormone which regulates carbohydrate, protein and fat metabolism in our body. Name the hormone and the gland which secretes it. Why is it important for us to have iodised salt in our diet?
Answer
The hormone is thyroxine, secreted by the thyroid gland. Iodised salt is important because iodine is required for the synthesis of thyroxine; its deficiency can lead to goitre, so iodised salt helps prevent this.
An object is placed at a distance of 10 cm from a convex mirror of focal length 15 cm. Find the position of the image formed by the mirror.
Answer
Given,
- Object distance () = -10 cm
- Focal length of the mirror () = 15 cm
Here, negative sign indicates that object is placed in front of the mirror.
By, using the mirror formula,
Hence, the image is formed behind the mirror at a distance of 6 cm from the mirror.
Show how you would connect three resistors each of resistance 6 Ω, so that the combination has a resistance of 9 Ω. Also justify your answer.
Answer
Given,
- Number of resistors = 3
- Resistance of each resistor () = 6 Ω
- Combined resistance () = 9 Ω
First combine two resistor in parallel combination and their effective resistance is given by,
Now, combine one more resistor in series with to get which is given by,
Justification : When two resistors are connected in parallel, their effective resistance decreases and adding another resistor in series increases the total resistance. By this arrangement, the overall resistance becomes exactly 9 Ω, as required.
Hence, combine two 6 Ω resistors in parallel and the third in series to give 9 Ω total resistance.
In the given circuit calculate the power consumed in watts in the resistor of 2 Ω:

Answer
From the figure,
- = 1 Ω
- = 2 Ω
- = 6 V
As, and are connected in series then their net resistance is given by,
By using Ohm's law the current drawn from the battery is given by,
Now, power consumed by 2 Ω is given by,
Hence, the power consumed in the resistor of 2 Ω is 8 W.
(a) Two magnetic field lines do not intersect each other. Why?
(b) How is a uniform magnetic field in a given region represented? Draw a diagram in support of your answer
Answer
(a) Two magnetic field lines never intersect each other because if they did, it would mean that at the point of intersection, the magnetic field has two different directions — one along each line but the magnetic field at any given point can have only one unique direction. Therefore, magnetic field lines do not cross each other.
(b) A uniform magnetic field means that the magnitude and direction of the magnetic field are the same at all points in the region.
It is represented by parallel, equally spaced straight lines as shown in below figure :

Write one chemical equation each for the chemical reaction in which the following have taken place :
(a) Change in colour
(b) Change in temperature
(c) Formation of precipitate
Mention colour change/temperature change (rise/fall)/compound precipitated along with equation.
Answer
(a) Change in colour reaction :
Observation : solution is blue; after the reaction the solution becomes pale green (due to ) and reddish-brown copper metal is deposited.
(b) Change in temperature (exothermic neutralisation) reaction :
Observation: The mixture becomes warm (temperature rises) because neutralisation releases heat.
(c) Formation of precipitate reaction:
Observation: A white precipitate of silver chloride (AgCl) is formed.
(a) The pH of a sample of tomato juice is 4.6. How is this juice likely to be in taste? Give reason to justify your answer.
(b) How do we differentiate between a strong acid and a weak base in terms of ion-formation in aqueous solutions?
(c) The acid rain can make the survival of aquatic animals difficult. How?
Answer
(a) The pH of tomato juice is 4.6, which is less than 7 and substances with pH below 7 are acidic in nature so, tomato juice should be sour in taste due to the presence of natural acids such as citric acid and ascorbic acid in it.
(b) A strong acid in water completely ionises to produce a large number of hydrogen ions (H+), for example HCl is a strong acid as it easily produces H+ ion as shown below :
HCl(aq) → H+(aq) + Cl-(aq)
A weak base, on the other hand, partially ionises in water and produces only a small number of hydroxide ions (OH-), for example NH4OH is a weak base as it ionises partially as shown below :
NH4OH(aq) ⇌ NH4+(aq) + OH-(aq)
Thus, the strength of an acid or base depends on the extent of ionisation in aqueous solution.
(c) Acid rain lowers the pH of river and lake water, making it more acidic. Aquatic animals, such as fish, cannot survive if the water becomes too acidic because it damages their gills, skin and eggs and disturbs enzyme activity necessary for life processes. Hence, acid rain makes the survival of aquatic life difficult.
(a) Why is respiratory pigment needed in multicellular organisms with large body size?
(b) Give reasons for the following :
- Rings of cartilage are present in the throat.
- Lungs always contain a residual volume of air.
- The diaphragm flattens and ribs are lifted up when we breathe in.
- Walls of alveoli contain an extensive network of blood vessels.
Answer
(a) In large multicellular organisms, all cells are not in direct contact with the external environment, so simple diffusion is not enough to supply oxygen to every cell. A respiratory pigment like haemoglobin is therefore needed to:
- pick up oxygen in the lungs,
- carry it through blood to body tissues,
- and help transport CO₂ back to the lungs.
(b)
- The rings of cartilage in the throat are present to keep the trachea (windpipe) open and prevent it from collapsing when there is less air pressure during breathing.
- The lungs always contain a residual volume of air so that there is continuous exchange of gases (oxygen and carbon dioxide) even between two breaths, preventing the lungs from collapsing completely.
- During inhalation, the diaphragm flattens and the ribs move upward and outward, which increases the volume of the chest cavity and reduces air pressure inside the lungs. This allows air to rush in from outside to fill the lungs.
- The walls of alveoli contain an extensive network of blood capillaries to provide a large surface area for the exchange of gases. Oxygen from the alveolar air diffuses into the blood, and carbon dioxide from the blood diffuses into the alveoli to be exhaled.
Define reflex action. With the help of a flow chart show the path of a reflex action such as sneezing.
Answer
A reflex action is an automatic, quick and involuntary response to a stimulus that does not involve the conscious part of the brain. It helps in protecting the body from harm and occurs through a pathway called the reflex arc.
Example – Reflex action in sneezing :
When dust or an irritant enters the nose, sensory nerves detect it and send signals that cause the muscles of the chest and diaphragm to contract suddenly, forcing air out — this is sneezing, which expels the irritant.
Flow chart showing the path of reflex action (Reflex Arc):
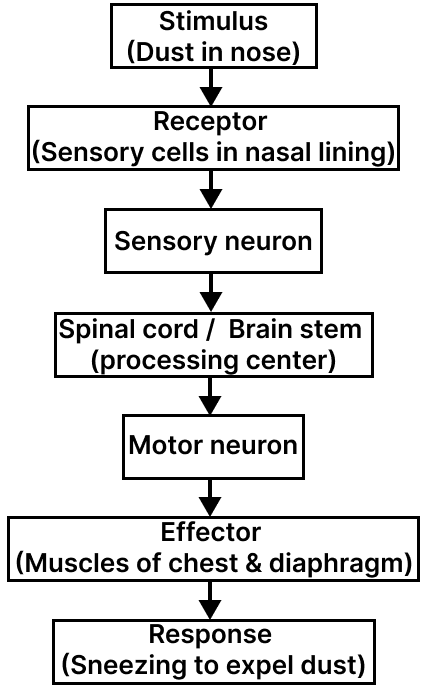
Study the diagram given below and answer the questions that follow :

(a) Name the defect of vision represented in the diagram. Give reason for your answer.
(b) List two causes of this defect.
(c) With the help of a diagram show how this defect of vision is corrected.
Answer
(a) The defect of vision shown in the diagram is Hypermetropia (long-sightedness).
Explanation : In the diagram, the rays of light coming from a near object (point N) converge to form the image behind the retina instead of on it which means the person can see distant objects clearly but cannot see nearby objects distinctly.
(b) Causes hypermetropia defect:
- The eyeball is too short, so the retina is closer to the eye lens.
- The focal length of the eye lens is too long (the lens becomes less convex), so it cannot bend light rays sufficiently to form the image on the retina.
(c) Hypermetropia is corrected by using a convex (converging) lens which converges the incoming light rays before they enter the eye so that they can be focused exactly on the retina.
Diagram showing correction for hypermetropic eye is given below :
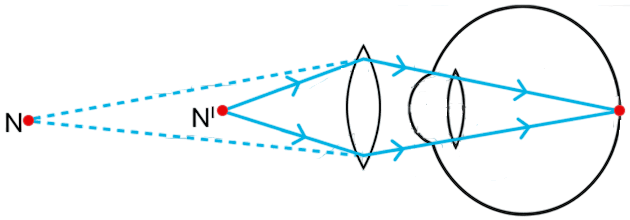
Name and state the rule to determine the direction of a :
(a) magnetic field produced around a current carrying straight conductor.
(b) force experienced by a current carrying straight conductor placed in a magnetic field which is perpendicular to it.
Answer
(a) The rule used to determine the direction of the magnetic field around a current-carrying straight conductor is the Right-Hand Thumb Rule.
Right-Hand Thumb Rule : If you hold the current-carrying conductor in your right hand such that your thumb points in the direction of the current, then the curl of your fingers around the conductor gives the direction of the magnetic field lines.
(b) The rule used to determine the direction of the force experienced by a current-carrying conductor placed in a perpendicular magnetic field is Fleming’s Left-Hand Rule.
Fleming’s Left-Hand Rule : If you stretch the thumb, forefinger, and middle finger of your left hand mutually perpendicular to each other such that the forefinger points in the direction of the magnetic field and the middle finger points in the direction of current, then the thumb will point in the direction of the force (motion) of the conductor.
Plants → Deer → Lion
In the given food chain, what will be the impact of removing all the organisms of second trophic level on the first and third trophic level? Will the impact be the same for the organisms of the third trophic level in the above food chain if they were present in a food web? Justify.
Answer
In the food chain:
Plants → Deer → Lion,
If all deer (second trophic level) are removed then :
- First trophic level (Plants):
Their population will increase, because there are no herbivores left to graze on them. - Third trophic level (Lions):
Their population will decrease and may die out, because their main food source (deer) is no longer available.
However, if lions were part of a food web instead of a single food chain, the impact would be less severe, because in a food web, lions have alternate prey options (for example, zebra or goat). Thus, the loss of one prey species (deer) would not immediately threaten their survival.
A gas 'X' which is a deadly poison is found at the higher levels of atmosphere and performs an essential function.
Name the gas and write the function performed by this gas in the atmosphere. Which chemical is linked to the decrease in the level of this gas? What measures have been taken by an international organization to check the depletion of the layer containing this gas?
Answer
The gas ‘X’ is ozone (O3).
It forms a layer in the stratosphere, called the ozone layer, which performs the essential function of absorbing harmful ultraviolet (UV) radiation from the Sun and thus protects living organisms from genetic damage and skin cancer.
The main chemicals responsible for the depletion of ozone are chlorofluorocarbons (CFCs), which were used in refrigerators, air conditioners, and aerosol sprays.
To check this depletion, the Montreal Protocol (1987) was signed by various countries as an international agreement to phase out the production and use of CFCs and other ozone-depleting substances.
(a) Define a homologous series of carbon compounds.
(b) Why is the melting and boiling points of C4H8 higher than that of C3H6 or C2H4?
(c) Why do we NOT see any gradation in chemical properties of a homologous series compounds?
(d) Write the name and structures of (i) aldehyde and (ii) ketone with molecular form C3H6O.
Answer
(a) A homologous series is a group of organic compounds having the same functional group, similar chemical properties and a constant difference of CH2 (14 atomic mass units) between successive members. Members show a gradual change in physical properties (like boiling point) but have similar chemical behaviour because of the same functional group.
(b) As carbon chain length increases (C2 → C3 → C4), the molecular mass and surface area increase. Larger molecules have stronger van der Waals (dispersion) forces between molecules, so more heat is needed to separate them. Therefore C4H8 (having a larger size) has higher melting/boiling points than C3H6 or C2H4.
(c) Chemical properties are mainly determined by the functional group, which is the same for all members of a homologous series. Because the reactive part is identical, they undergo similar chemical reactions (no gradual change) but Physical properties (boiling point, melting point) show gradual change due to molecular size.
(d)
- Propanal (also called propionaldehyde)
Structural formula : CH3—CH2—CHO
- Propanone (commonly called acetone)
Structural formula : CH3—CO—CH3
(a) Write the name and structure of an organic compound 'X' having two carbon atoms in its molecule and its name is suffixed with '— ol'.
(b) What happens when 'X' is heated with excess concentrated sulphuric acid at 443 K? Write chemical equation for the reaction stating the conditions for the reaction. Also state the role played by concentrated sulphuric acid in the reaction.
(c) Name and draw the electron dot structure of hydrocarbon produced in the above reaction.
Answer
(a) An organic compound 'X' with two carbon atoms whose name ends with "-ol" is ethanol.
- Molecular formula : C2H5OH.
- Structural formula : CH3—CH2—OH.
(b) This gives dehydration (elimination) of ethanol to form ethene (C2H4) as shown below :
Role of conc. H2SO4 :
- Acts as a dehydrating agent (removes water).
- Acts as an acid catalyst, helping protonate ethanol and promote loss of water to form the alkene.
(c)
- Name of the compound formed : Ethene (also called ethylene)
- Molecular formula : C2H4
Electron-dot (Lewis) structure of ethene is shown below :

(a) Name three techniques/devices used by human females to avoid pregnancy. Mention the side effects caused by each.
(b) What will happen if in a human female
- fertilisation takes place,
- an egg is not fertilised?
Answer
(a) Three techniques/devices used by human females to avoid pregnancy and their side effects :
1. Oral contraceptive pills :
- Function : These pills contain hormones that prevent ovulation (release of the egg).
- Side effects : May cause nausea, weight gain, mood changes and irregular menstrual cycles.
2. Copper-T :
- Function : A small device inserted into the uterus that prevents the fertilised egg from implanting in the uterine wall.
- Side effects : Can cause abdominal pain, excessive menstrual bleeding and risk of infection.
3. Surgical method (Tubectomy):
- Function : The fallopian tubes are cut and tied to prevent the egg from reaching the uterus.
- Side effects : May cause pain, infection or complications related to surgery; it is a permanent method.
(b) What happens if:
If fertilisation takes place then the zygote formed in the oviduct undergoes repeated cell divisions, travels to the uterus, and implants in the uterine wall. It develops into an embryo and later a foetus, resulting in pregnancy.
If the egg is not fertilised then the unfertilised egg, along with the thickened uterine lining, is shed out of the body through the vagina in the form of menstrual bleeding, called menstruation.
(a) Draw a diagram showing spore formation in Rhizopus and label the (i) reproductive and (ii) non-reproductive parts. Why does Rhizopus not multiply on a dry slice of bread?
(b) Name and explain the process by which reproduction takes place in Hydra.
Answer
(a) Diagram showing spore formation in Rhizopus :

- Reproductive part : Sporangium (produces spores).
- Non-reproductive parts : Hyphae and Rhizoids (absorb nutrients and anchor the fungus).
Rhizopus (a fungus) does not multiply on a dry slice of bread because moisture is essential for spore germination so in the absence of moisture, spores remain inactive and cannot grow into new hyphae.
(b) Hydra reproduces by a process called budding.
- Budding : A small bud develops on the body wall of the parent hydra due to repeated cell division at a specific site. This bud grows into a small hydra and, when fully mature, detaches from the parent to live as an independent organism.
(a) Define electric power. Express it in terms of potential difference (V) and resistance (R).
(b) An electric oven is designed to work on the mains voltage of 220 V. This oven consumes 11 units of electrical energy in 5 hours. Calculate :
- power rating of the oven
- current drawn by the oven
- resistance of the oven when it is red hot
Answer
(a) Electric power is the rate at which electrical energy is consumed or converted into other forms of energy such as heat, light or mechanical work in an electrical circuit.
It is given by :
where
- = electric power,
- = potential difference,
- = current
Using Ohm’s law (),
Thus, electric power can also be expressed in terms of potential difference (V) and resistance (R) as:
(b) Given,
- Mains voltage () = 220 V
- Electrical energy consumed by the oven () = 11 units
- Time duration for operating the oven () = 5 hours
- As
1 unit = 1 KWh
Then
= 11 units = 11 KWh
Also,
Power rating of the oven is given by,
Hence, the power rating of the oven is 2200 W.
- Current drawn by the oven is given by,
Hence, the current drawn by the oven is 10 A.
- Resistance of the oven is given by Ohm's law,
Hence, the resistance of the oven when it is red hot is 22 Ω.
(a) Write the relation between resistance and electrical resistivity of the material of a conductor in the shape of cylinder of length and area of cross-section . Hence derive the SI unit of electrical resistivity.
(b) The resistance of a metal wire of length 3 m is 60 Ω. If the area of cross-section of the wire is 4 × 107 m2, calculate the electrical resistivity of the wire.
(c) State how would electrical resistivity be affected if the wire (of part 'b') is stretched so that its length is doubled. Justify your answer.
Answer
(a) Resistance of a cylindrical conductor is given by,
Then,
Hence, SI unit of ρ is Ω.m.
(b) Given,
- Resistance of the wire () = 60 Ω
- Length of the wire () = 3 m
- Area of cross-section of the wire () = 4 × 10-7 m2
As, resistance of a cylindrical conductor is given by,
where is electrical resistivity of the wire.
Then,
Hence, the electrical resistivity of the wire is 8 × 10-6 Ωm.
(c) When the wire is stretched, its length increases and area of cross-section decreases, but resistivity (ρ) depends only on the nature of the material and temperature, not on its dimensions.
Hence, electrical resistivity remains unchanged if the material and temperature are the same, even though the resistance will increase.
The metals produced by various reduction processes are not very pure. They contain impurities, which must be removed to obtain pure metals. The most widely used method for refining impure metals is electrolytic refining.
(a) What is the cathode and anode made of in the refining of copper by this process ?
(b) Name the solution used in the above process and write its formula.
(c) How copper gets refined when electric current is passed in the electrolytic cell?
OR
(c) You have two beakers 'A' and 'B' containing copper sulphate solution. What would you observe after about 2 hours if you dip a strip of zinc in beaker 'A' and a strip of silver in beaker ‘B’? Give reason for your observations in each case.
Answer
(a) In the electrolytic refining of copper:
- Cathode → A thin strip of pure copper
- Anode → A thick plate of impure copper
(b)
The electrolyte (solution used) is acidified copper sulphate solution (CuSO4).
(c) When electric current is passed through the electrolyte then
- Copper from the anode (impure copper) dissolves into the solution as Cu2+ ions.
- These Cu2+ ions from the electrolyte are deposited on the cathode as pure copper.
- The impurities (like Ag, Au, Pt) settle down below the anode as anode mud.
Chemical reactions:
- At anode : Cu (impure) → Cu2++ 2e-
- At cathode : Cu2+ + 2e- → Cu (pure)
Thus, pure copper is deposited at the cathode and impurities are left behind or fall as anode mud.
OR
(c)
- Beaker A : Zinc strip in copper sulphate solution
Zinc is more reactive than copper so, zinc displaces copper from copper sulphate solution.
Observation : Reddish-brown deposit of copper forms on zinc strip and the blue colour of the solution fades due to decrease in Cu2+ ions.
Reaction : Zn + CuSO4 → ZnSO4 + Cu
- Beaker B : Silver strip in copper sulphate solution
Silver is less reactive than copper so, no displacement reaction takes place.
Observation : No change in colour of the solution and no deposit on the silver strip.
Mendel worked out the rules of heredity by working on garden pea using a number of visible contrasting characters. He conducted several experiments by making a cross with one or two pairs of contrasting characters of pea plant. On the basis of his observations he gave some interpretations which helped to study the mechanism of inheritance.
(a) When Mendel crossed pea plants with pure tall and pure short characteristics to produce F1 progeny, which two observations were made by him in F1 plants?
(b) Write one difference between dominant and recessive trait.
(c) In a cross with two pairs of contrasting characters
RRYY (Round Yellow) X rryy (Wrinkled Green)
Mendel observed 4 types of combinations in F2 generation. By which method did he obtain F2 generation? Write the ratio of the parental combinations obtained and what conclusions were drawn from this experiment.
OR
(c) Justify the statement : "It is possible that a trait is inherited but may not be expressed."
Answer
(a) When Mendel crossed pure tall (TT) and pure short (tt) pea plants then two observation were :
- All F1 plants were tall, showing that the tall trait dominates over the short one.
- The short trait reappeared in the F2 generation, showing that it was not lost but only masked in the F1 generation.
(b)
| Dominant Trait | Recessive Trait |
|---|---|
| Expressed in the F1 generation, even when one allele is present. | Expressed only when both alleles are of the same type (homozygous). |
| Example: Tallness in pea plant (T) | Example: Dwarfness in pea plant (t) |
(c) Mendel performed a dihybrid cross between RRYY (Round Yellow) and rryy (Wrinkled Green) pea plants and he obtained the F2 generation by self-pollinating the F1 hybrids (RrYy × RrYy).
Observations in F2 generation:
- Four types of combinations appeared:
- Round Yellow (RRYY, RrYY, RRYy, RrYy)
- Round Green (RRyy, Rryy)
- Wrinkled Yellow (rrYY, rrYy)
- Wrinkled Green (rryy)
Ratio of parental combinations:
- Parental combinations (Round Yellow + Wrinkled Green) → 9 + 1 = 10 parts
- New (recombinant) combinations (Round Green + Wrinkled Yellow) → 3 + 3 = 6 parts
So, the overall phenotypic ratio = 9 : 3 : 3 : 1.
Conclusion drawn:
- The inheritance of one pair of contrasting traits does not affect the inheritance of another pair.
- This led to Mendel’s Law of Independent Assortment.
OR
(c) It is possible that a trait is inherited but may not be expressed because some traits are recessive and remain masked by dominant traits in the F1 generation.
Example:
- When a pure tall (TT) pea plant is crossed with a pure dwarf (tt) plant, all F1 plants are tall (Tt).
- The dwarfness trait is inherited from the parent but not expressed in F1 due to the dominance of tallness.
- The trait reappears in F2 when two recessive alleles come together (tt).
Hence, a trait may be present in the genes but hidden in expression.
Study the data given below showing the focal length of three concave mirrors A, B and C and the respective distances of objects placed in front of the mirrors:
| Case | Mirror | Focal Length (cm) | Object Distance (cm) |
|---|---|---|---|
| 1 | A | 20 | 45 |
| 2 | B | 15 | 30 |
| 3 | C | 30 | 20 |
(a) In which one of the above cases the mirror will form a diminished image of the object? Justify your answer.
(b) List two properties of the image formed in case 2.
(c) What is the nature and size of the image formed by mirror C? Draw ray diagram to justify your answer.
OR
(c) An object is placed at a distance of 18 cm from the pole of a concave mirror of focal length 12 cm. Find the position of the image formed in this case.
Answer
(a) Case 1 (Mirror A) will form a diminished image.
Explanation : The object (45 cm) is beyond C (40 cm) and for a concave mirror an object beyond C produces a real, inverted and diminished image located between f and C.
(b) Two properties of the image formed in case 2 (Mirror B) where object is at C :
- The image is real and inverted.
- The image is of the same size as the object and is formed at C (30 cm) on the other side of the mirror.
(c) For mirror C (f = 30 cm, object at 20 cm) :
- Nature of image : Virtual and erect (since the object lies between pole and focus).
- Size of image: Magnified (appears larger than the object).
- Where formed : Behind the mirror (cannot be projected on a screen).
The ray diagram of the above arrangement is shown below :
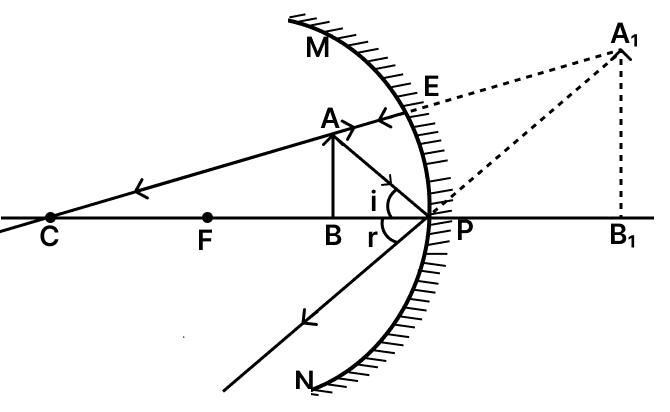
OR
(c)
Given,
- Object distance () = -18 cm
- Focal length of the mirror () = -12 cm
Here, negative sign indicates that object is in front of the mirror and parallel rays after reflection converges in front of the mirror.
Let, image distance be ''.
From mirror formula,
Hence, the image is formed at a distance of 36 cm in front of the mirror.