The product formed at the cathode in electroplating of an article with Silver is:
- Hydrogen gas
- Silver ions
- Silver atoms
- Oxygen gas
Answer
Silver atoms
Reason — Ag+ ions from the electrolyte gain electrons (reduction) and are deposited as silver atoms on the article.
Ag+ + e- ⟶ Ag
Ionisation potential is maximum in:
- Alkaline earth metals
- Halogens
- Inert gases
- Alkali metals
Answer
Inert gases
Reason — Ionisation potential (or ionisation energy) is the energy required to remove the outermost electron from an atom in the gaseous state. It generally increases from left to right across a period (because effective nuclear charge increases) and decreases down a group (because atomic size and shielding increase).
Noble gases have completely filled valence shells and very small atomic radii within their periods; therefore, much more energy is needed to remove an electron from them, giving them the maximum ionisation energy among the groups listed.
If the RMM of carbon monoxide is 28, then its vapour density is:
- 7
- 56
- 14
- 88
Answer
14
Reason —
Given,
The relative molecular mass(RMM) of carbon monoxide = 28.
The vapour density of carbon monoxide = ?
Vapour density =
Vapour density = = 14
Hence, the vapour density of carbon monoxide is 14.
A triple covalent bond is present in:
- Methane
- Ammonia
- Nitrogen
- Chlorine
Answer
Nitrogen
Reason — A triple covalent bond involves three shared pairs of electrons between two atoms. Each nitrogen atom shares 3 pairs of electrons, forming a triple bond.
Assertion (A): Acetic acid has four hydrogen atoms in its molecule but its basicity is one.
Reason (R): Acetic acid has only one replaceable hydrogen atom.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true and R is the correct explanation of A.
Reason — The molecule CH3COOH possesses four hydrogen atoms. Three are attached to the methyl group (CH3) and are non-ionisable, while the single hydrogen in the –COOH (carboxylic) group is ionisable. Because only one hydrogen can be released as an H+ ion, the basicity of acetic acid is 1. Thus both the Assertion and the Reason are correct, and the Reason properly explains the Assertion.
The hydroxide which is soluble in excess of NH4OH is:
- Ferric hydroxide
- Lead hydroxide
- Copper hydroxide
- Calcium hydroxide
Answer
Copper hydroxide
Reason — Copper hydroxide reacts with NH4OH to give pale blue precipitate of Cu(OH2). When excess NH4OH is added, Cu(OH)2 dissolves due to the formation of a soluble deep blue complex.
The main components of brass are:
- Copper and tin
- Copper and iron
- Copper and lead
- Copper and zinc
Answer
Copper and zinc
Reason — Alloy brass consist of 60-70% of copper and 40-30 % of zinc.
The drying agent used to dry Ammonia is:
- Concentrated Sulphuric acid
- Calcium oxide
- Sulphurous acid
- Calcium hydroxide
Answer
Calcium oxide
Reason — The drying agent used to dry Ammonia is quicklime or calcium oxide (CaO). Other drying agents like conc. sulphuric acid and sulphurous acid are not used, as ammonia being basic, reacts with them. Calcium hydroxide (slaked lime) is slightly basic, but less effective than calcium oxide in drying. It may also partially react with ammonia.
The percentage of nitrogen present in urea (NH2)2CO is: [R.A.M. of N = 14, C= 12, O = 16, H = 1]
- 23.36
- 46.67
- 19.35
- 43.87
Answer
46.67
Reason —
Molar mass of urea (CON2H4) = 12 + 16 + 28 + 4 = 60 g
Molar mass of nitrogen (N2) = 2 x 14 = 28 g
60 g urea has mass of nitrogen = 28 g
∴ 100 g urea will have mass
=
= 46.67%
A few drops of universal indicator are added to colourless solution A, B, C and D with pH 2, 10, 9 and 7 respectively. Which of the following test tube is labelled with incorrect colour ?
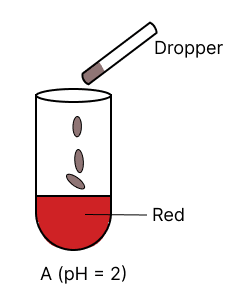
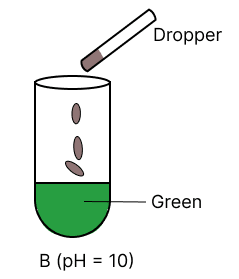
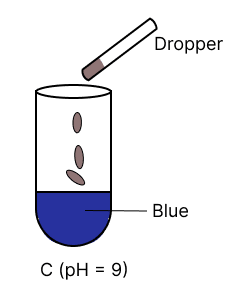
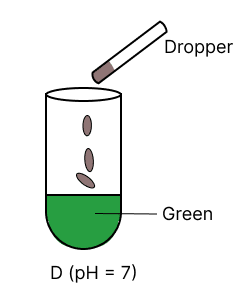
Answer
pH 10
Reason —
Universal indicator colour range.
| pH | Colour |
|---|---|
| 0 - 3 | Red |
| 4 - 6 | Orange to yellow |
| 7 | Green |
| 8 - 11 | Blue |
| 12 - 14 | Purple/ Violet |
Option 2 is labelled incorrectly, it should be labelled as blue.
Assertion (A): Hydrogen is a neutral gas but it cannot be dried by concentrated sulphuric acid.
Reason (R): The gas reacts with this acid.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true, and R is the correct explanation of A.
Reason — Hydrogen is neutral (neither acidic nor basic), and while concentrated H2SO4 is a drying agent, it cannot be used for drying hydrogen because concentrated H2SO4 is a strong oxidizing agent and may oxidize hydrogen, especially under certain conditions, which makes it unsafe and unsuitable. Hence, the assertion (A) is true.
Hydrogen gas reacts with concentrated H2SO4 because sulphuric acid is a strong oxidizing agent. Hence, the reason (R) is true and it explains why hydrogen cannot be dried with concentrated H2SO4.
The organic compound prepared when calcium carbide reacts with water:
- Methane
- Ethane
- Acetylene
- Ethene
Answer
Acetylene
Reason — In the laboratory preparation of Acetylene, calcium carbide reacts with water to give acetylene and calcium hydroxide.
The IUPAC name of acetylene is:
- Propyne
- Ethene
- Propane
- Ethyne
Answer
Ethyne
Reason — Acetylene has the chemical formula C2H2. According to IUPAC nomenclature, alkynes are named with the suffix “-yne” and have 2 carbon atoms, so the prefix is “eth-”. Hence. the name 'Ethyne'
A strong electrolyte is:
- Alcohol
- Potassium hydroxide
- Ammonium hydroxide
- Glucose
Answer
Potassium hydroxide
Reason — When dissolved in water, a strong electrolyte entirely dissociates into ions, making it an excellent electrical conductor.
| Compound | Type | Electrolyte strength | Explanation |
|---|---|---|---|
| Alcohol | Covalent compound | Non-electrolyte | Does not ionize in water |
| Potassium hydroxide | Ionic compound (Strong base) | Strong electrolyte | Completely dissociates into K+ and OH- in water |
| Ammonium hydroxide | Weak base | Weak electrolyte | Partially ionizes in water |
| Glucose | Covalent organic compound | Non-electrolyte | Dissolves but does not form ions in water |
The salt which in solution gives a pale green precipitate with sodium hydroxide solution and a white precipitate with barium chloride solution is:
- Iron (III) sulphate
- Iron (II) sulphate
- Iron (II) chloride
- Iron (III) chloride
Answer
Iron (II) sulphate
Reason — Iron (II) sulphate (FeSO4) reacts with NaOH to give pale green precipitate of ferrous hydroxide.
The sulphate ion (SO42-) from FeSO4 reacts with BaCl2 to form, white precipitate of BaSO4
Ba2+ + SO42- ⟶ BaSO4
The figure given below illustrates the apparatus used in the laboratory preparation of nitric acid.

(a) Name A (a liquid), B (a solid) and C (a liquid). (Do not give the formulae).
(b) Write a balanced chemical equation for the above preparation.
(c) Why is an all glass apparatus used ?
(d) The acid prepared is yellow in colour. Why?
(e) How is this colour removed?
Answer
(a)
A — Concentrated sulphuric acid,
B — Sodium nitrate or potassium nitrate
C — Nitric acid.
(b)
(c) All glass apparatus is used in the laboratory preparation of nitric acid since the vapours of nitric acid being highly corrosive attack rubber, cork, etc.
(d) Pure nitric acid [HNO3] is colourless and unstable and decomposes slightly even at ordinary temperatures and in the presence of sunlight. The decomposition results in formation of reddish brown nitrogen dioxide [NO2] which remains dissolved in the acid thus imparting a slight yellowish brown colour.
(e) If dry air or CO2 is bubbled through the yellow acid, the latter turns colourless because it drives out NO2 from warm acid which is further oxidised to nitric acid.
Match Column A with Column B.
| Column A | Column B | ||
|---|---|---|---|
| (a) | Acid Salt | (i) | Black in colour |
| (b) | Manganese dioxide | (ii) | Brown ppt. |
| (c) | Lead hydroxide | (iii) | Hydrogen chloride |
| (d) | Ferric hydroxide | (iv) | Calcium hydrogen carbonate |
| (e) | Polar compound | (v) | Soluble in excess sodium hydroxide |
Answer
| Column A | Column B | ||
|---|---|---|---|
| (a) | Acid Salt | (iv) | Calcium hydrogen carbonate |
| (b) | Manganese dioxide | (i) | Black in colour |
| (c) | Lead hydroxide | (v) | Soluble in excess sodium hydroxide |
| (d) | Ferric hydroxide | (ii) | Brown ppt. |
| (e) | Polar compound | (iii) | Hydrogen chloride |
Complete the following by choosing the correct answers from the bracket:
(a) HCl in the liquefied form is ............... [neutral / acidic]
(b) Organic compounds are generally soluble in ...............[Water / Organic solvents]
(c) An inert electrode used in electrolysis of copper sulphate solution is ............... [Copper/platinum]
(d) Hydrocarbons having triple bond is ............... [alkenes/alkynes]
(e) An acidic gas gives dense white fumes of ............... [NH4OH/NH4C1] with ammonia.
Answer
(a) HCl in the liquefied form is neutral
(b) Organic compounds are generally soluble in Organic solvents.
(c) An inert electrode used in electrolysis of copper sulphate solution is platinum.
(d) Hydrocarbons having triple bond is alkynes.
(e) An acidic gas gives dense white fumes of NH4C1 with ammonia.
Identify the following:
(a) The compound formed by carbon and hydrogen only.
(b) A substance that do not conduct electricity in molten or aqueous state.
(c) The energy released when an atom in the gaseous state accepts an electron to form an anion.
(d) The name of the process by which aluminium is obtained from alumina.
(e) The bond formed by mutual sharing of a shared pair of electrons.
Answer
(a) Hydrocarbon
(b) Non-electrolyte (Covalent compound)
(c) Electron affinity
(d) Hall-Héroult process (electrolytic reduction)
(e) Covalent bond
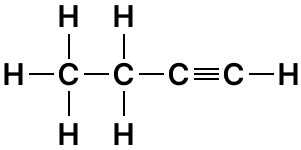
(a) Draw the structure formula for the following :
- 2-pentanal
- 2-methyl butanol
- 1-butyne
(b) Name the following organic compound in IUPAC system :
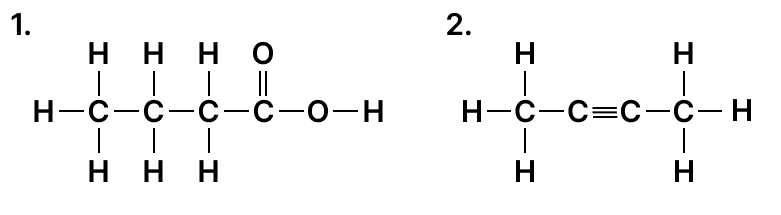
Answer
(a)
2-pentanal

2-methyl butanol
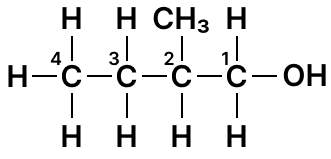
1-butyne
(b)
- Butanoic acid
- But-2-yne
Arrange the following as per the instruction given in the brackets:
(a) Na, K, Cl, Si, S (increasing order of ionisation potential)
(b) Be, Li, F, C, B, N, O (increasing order of non-metallic character)
Answer
(a) K < Na < Si < S < Cl
(b) Li < Be < B < C < N < O < F
Identify the Anion present in each of the following compounds :
(a) When Silver nitrate solution is added to a solution of compound B, a white precipitate soluble in ammonium hydroxide solution is formed.
(b) When dilute Sulphuric acid is added to compound D, a gas is produced which turns lime water milky and also turns acidified potassium dichromate solution green.
Answer
(a) Chloride ion (Cl-)
(b) Sulphite ion (SO32-)
Write the products and balance the equation.
(a) C + Conc. ΗΝΟ3 ⟶
(b) Na2SO3 + HCl ⟶
(c) C2H6 + O2 ⟶
Answer
(a) C + 4ΗΝΟ3 ⟶ CO2 + 2H2O + 4NO2
(b) Na2SO3 + 2HCl ⟶ 2NaCl + H2O + SO2
(c) 2C2H6 + 7O2 ⟶ 4CO2 + 6H2O
Give balanced chemical equations for the following:
(a) Laboratory Preparation of Ethylene.
(b) Preparation of Ethanol by hydrolysis of alkyl halide.
(c) Ethene reacting with bromine.
Answer
(a)
(b)
(c)
What is the role played by the following substances in the extraction of Aluminium ?
(a) Caustic soda
(b) Fluorspar
Answer
(a) Caustic soda is used in the Bayer purification of bauxite. Bauxite is heated under pressure with conc. caustic soda (NaOH) for 2 to 8 hours. Hot, concentrated NaOH dissolves the amphoteric alumina present in the ore, forming soluble sodium aluminate:
The insoluble impurities mainly Fe2O3 and SiO2 are left behind and are referred to as Red mud.
(b) Fluorspar is added to the electrolyte mixture as it lowers the fusion point of the mixture i.e., the mixture fuses around 950°C instead of 2050°C.
Calculate:
A gas cylinder is filled with hydrogen and it holds 50 g of gas. The same cylinder holds 200 g of gas X and 500 g of gas Y under same temperature and pressure conditions. Calculate the vapour density of gas X and molecular mass of gas Y.
Answer
Given,
At same temperature and pressure Mass of hydrogen = 50 g
Mass of gas X = 200 g
Mass of gas Y = 500 g
V.D. of gas X = = = 4
V.D. of gas Y = = = 10
Molecular weight = 2 x V.D. = 2 x 10 = 20 a.m.u.
∴ The vapour density of gas X is 4 and Molecular mass of gas Y is 20 a.m.u.
Explain the following :
(a) HCl gas dissolved in toluene does not affect litmus.
(b) An inverted funnel is used to dissolve Ammonia gas in water.
(c) A bottle of liquor ammonia should be opened very carefully.
Answer
(a) HCl gas is covalent in nature and does not ionise in non-polar solvents like toluene. Since, there is no ionisation of HCl, so no hydronium (H3O+) ions are produced. As, acidity depends on the presence of these ions, neither blue nor red litmus shows any colour change; the solution is neutral to litmus.
(b) The mouth of the inverted funnel provides a larger surface area over which ammonia gas can come into contact with water, so the gas dissolves rapidly. At the same time, keeping the funnel rim just below the water surface allows air to enter if the pressure inside the apparatus falls, preventing water from being sucked back into the flask that contains the gas.
(c) Liquor ammonia (aqueous NH3) is stored under considerable internal pressure owing to the high volatility of ammonia. The bottle is first cooled in ice-cold water. The cooling lowers the vapour pressure, so when the stopper is removed the contents do not spurt out and there is no sudden escape of ammonia gas.
The following questions are pertaining to the laboratory preparation of Ammonia gas.
(a) Write a balanced chemical equation for its preparation mentioning the conditions required.
(b) Why is a higher ratio by weight of the alkali used?
(c) How is Ammonia gas collected?
Answer
(a) 2NH4Cl + Ca(OH)2 ⟶ CaCl2 + 2H2O + 2NH3↑
Finely grounded mixture of ammonium chloride and calcium hydroxide in excess is taken in a round-bottom flask fitted in a slanting position and gently heated to liberate ammonia gas.
(c) Ammonia gas is collected in inverted gas jars by the downward displacement of air.
Name a probable cation present based on the following observations:
(a) Green precipitate insoluble in Ammonium Hydroxide.
(b) Gelatinous white precipitate soluble in excess of NaOH solution
Answer
(a) Fe2+ (ferrous ion)
(b) Zn2+ (zinc ion)
Prepare lead sulphate from lead chloride.
Answer
Preparation of lead sulphate from lead chloride:
- Dissolve lead chloride (PbCl2) in hot water as the salt is only sparingly soluble in cold water but dissolves better when heated.
- While the solution is still hot, slowly add a dilute solution of sulphuric acid with constant stirring.
- Allow the mixture to cool, then filter to collect the precipitate.
- Wash the precipitate with distilled water and finally dry it.
What do you see in each of the following reactions:
(a) When excess Ammonia gas is passed through an aqueous solution of Zinc Nitrate.
(b) Copper Sulphate crystals are heated strongly.
(c) Sodium hydroxide is added to Copper Sulphate solution in excess.
Answer
(a) When Ammonia gas is passed through an aqueous solution of Zinc Nitrate:
- A white, gelatinous precipitate of zinc hydroxide, Zn(OH)2, first appears.
Zn(NO3)2 + 2NH4OH ⟶ 2NH4NO3 + Zn(OH)2 ↓ - On passing excess ammonia, the precipitate dissolves and the solution becomes clear and colourless owing to the formation of the soluble tetra-amminezinc(II) complex, [Zn(NH3)4]2+.
(b) The blue crystals of hydrated copper sulphate (CuSO4·5H2O) lose their water of crystallisation, giving off steam and turning into a white powder of anhydrous CuSO4:
(c) Copper(II) sulphate reacts with NaOH to form a blue precipitate of copper(II) hydroxide (Cu(OH)2), which is insoluble in excess NaOH.
CuSO4 + 2NaOH ⟶ Cu(OH)2 ↓ + Na2SO4
Given below is a sketch of an electrolytic cell used in the extraction of aluminium.

(a) What is the substance of which the electrodes A and B are made?
(b) At which electrode (A or B) is the aluminium formed?
(c) What are the two aluminium compounds in the electrolyte C?
Answer
(a) Electrodes A and B are made of graphite (carbon).
(b) Aluminium is formed at cathode i.e., electrode A.
(c) Two aluminium compounds in the electrolyte C are alumina [Al2O3] and Cryolite [Na3AlF6]
Define
(a) Electron affinity.
(b) Catenation.
Answer
(a) The amount of energy released while converting a neutral gaseous isolated atom into a negatively charged gaseous ion (anion) by the addition of electron is called Electron Affinity (E.A.).
(b) The property of self linking of atoms of an element through covalent bonds in order to form straight chains, branched chains and cyclic chains of different sizes is known as catenation.
1250 cc of oxygen was burnt with 300 cc of ethane (C2H6). Calculate the volume of the unused oxygen and the volume of the carbon dioxide formed:
2C2H6 + 7O2 ⟶ 4CO2 + 6H2O
Answer
[By Gay Lussac's law]
2 Vol. of C2H6 requires 7 Vol. of oxygen
∴ 300 cc C2H6 will require x 300
= 1050 cc of Oxygen
Hence, unused oxygen = 1250 - 1050 = 200 cc
Similarly,
2 Vol. of C2H6 produces 4 Vol. of carbon dioxide
∴ 300 cc C2H6 produces x 300
= 600 cc of Carbon dioxide
Hence, carbon dioxide produced = 600 cc.
Write a balanced reaction where sulphuric acid shows the following properties:
(a) Oxidising agent
(b) Non Volatile Acid
(c) Dehydrating agent
Answer
(a) C + 2H2SO4 ⟶ CO2 + 2H2O + 2SO2 ↑
(b) NaCl + H2SO4 ⟶ NaHSO4 + HCl
(c)
Choose the answer which fits the description from the list given below:
[CaO, CO2, NaOH, Fe(OH)2, CO, ZnO]
(a) A base insoluble in water.
(b) An oxide which is yellow when hot and white when cold.
(c) A neutral oxide
Answer
(a) Fe(OH)2
(b) ZnO
(c) CO
During the electrolysis of acidulated water using platinum electrodes:
(a) State the volume ratio of the products formed at electrodes.
(b) Is it an example of catalysis?
(c) Write the reaction occurring at the anode
Answer
(a) Hydrogen and oxygen are obtained in the volume ratio 2 : 1 (H2 : O2).
(b) Water in pure state consists almost entirely of molecules. It is a polar covalent compound and can form ions when traces of dilute sulphuric acid is added. As dilute sulphuric acid catalyses this ionisation, hence this electrolysis of acidified water is considered as an example of catalysis.
(c) Reaction at anode:
4OH- - 4e- ⟶ 4OH
4OH ⟶ 2H2O + O2
Name the catalyst required for the following processes:
(a) Contact process
(b) Ostwald process
(c) Haber process
Answer
(a) Vanadium pentoxide (V2O5)
(b) Platinum
(c) Finely divided Iron (Fe)
An organic compound contains: H = 6.32 %, N = 17.76%. In the vapour state, this compound is 39.5 times as heavy as the same volume of hydrogen.
(a) Find the molecular formula of the compound. (At wt: H = 1 N = 14 )
(b) Calculate the number of hydrogen atoms in one mole of this compound.
Answer
Given:
Percentage of H = 6.32%
Percentage of N = 17.76%
Percentage of carbon = 100 - (6.32 + 17.76) = 75.92 %
Atomic weights: H = 1, N = 14
(a)
| Element | % composition | At. wt. | Relative no. of atoms | Simplest ratio |
|---|---|---|---|---|
| Hydrogen | 6.32 | 1 | = 6.32 | = 5 |
| Nitrogen | 17.76 | 14 | = 1.27 | = 1 |
| Carbon | 75.92 | 12 | = 6.32 | = 5 |
Simplest ratio of whole numbers = H : N : C = 5 : 1 : 5
Hence, empirical formula is C5H5N
Empirical formula weight = (5 × 12) + (5 × 1) + (1 × 14) = 60 + 5 + 14 = 79 g/mol
V.D. = 39.5
Molecular weight = 2 x V.D. = 2 x 39.5 = 79
∴ Molecular Formula = n[E.F.] = 1[C5H5N] = C5H5N
(b) From the formula C5H5N, there are 5 hydrogen atoms per molecule.
In 1 mole of the compound the number of molecules = 6.022 × 1023
∴ Total hydrogen atoms = 5 × (6.022 × 1023) = 3.011 × 1024 hydrogen atoms.
An element X has atomic number 12. Answer the following questions:
(a) State the period and group to which it belongs.
(b) Write the formula of the compound formed between X and the second member of the halogen group
Answer
(a) Atomic number 12 corresponds to the electronic configuration 2, 8, 2.
- Period: three occupied shells → Period 3.
- Group: two valence electrons → Group 2 (alkaline earth metals).
(b) Second member of halogen group is chlorine. Element X has valency 2, and Chlorine has valency 1. So, the formula will be MgCl2.
A basic gas is dissolved in water. Draw the electron dot diagram of the ions formed.
Answer
The ion formed is Hydroxyl ion. Its electron dot diagram is shown below:
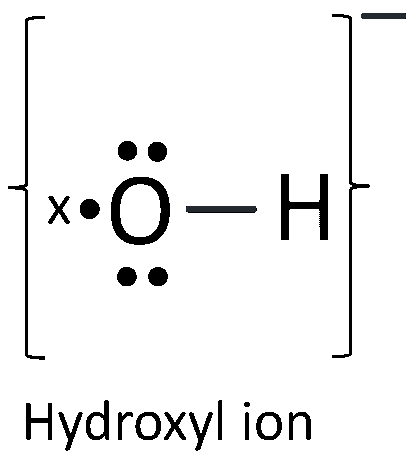
Distinguish between the following as directed:
(a) Sodium Sulphite and Sodium Sulphate by using dilute HCl.
(b) Ammonium chloride and Sodium chloride by using Calcium hydroxide.
(c) Lead nitrate and silver nitrate by using HCl.
Answer
(a) When Sodium sulphite reacts with HCl, effervescence occurs and a pungent gas (SO2) is evolved that turns acidified potassium dichromate paper from orange to green.
Na2SO3 + 2HCl ⟶ 2NaCl + H2O + SO2 ↑
Whereas, Sodium sulphate (Na2SO4) gives no visible reaction with HCl and no gas is evolved.
(b) Ammonium Chloride (NH4Cl) reacts with Ca(OH)2 to release ammonia gas (NH3) which has pungent smell, turns red litmus blue.
2NH4Cl + Ca(OH)2 ⟶ CaCl2 + 2H2O + 2NH3↑
Whereas, sodium chloride shows no reaction with Calcium hydroxide.
(c) Lead Nitrate (Pb(NO3)2) reacts with HCl, forms a white precipitate of lead chloride (PbCl2) which is soluble in hot water.
Pb(NO3)2 + 2HCl ⟶ PbCl2 ↓ + 2HNO3
However, when Silver nitrate reacts with HCl, it forms a white precipitate of silver chloride (AgCl) which is insoluble in hot water
AgNO3 + HCl ⟶ AgCl ↓ + HNO3
The formulae of some organic compounds are:
A

B

C

D
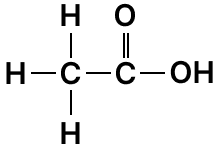
(a) Write an equation for the preparation of A using B.
(b) Name the compound formed when B and D reacts in presence of a mineral acid.
(c) Write an equation for the preparation of C.
Answer
(a) By heating ethyl alcohol with concentrated H2SO4 at 170°C.
(b) Ethyl ethanoate
(c)