(a) Give a chemical test to identify the following gases :
- Ammonia
- Sulphur dioxide
- Hydrogen chloride
- Chlorine
- Carbon dioxide
- Oxygen
- Hydrogen
(b) Select a basic gas mentioned in Q.1(a). How is the basic nature verified?
(c) Select acidic gases from the gases mentioned in Q.1(a). How is the acidic nature verified?
(d) State the gas responsible for the bleaching action?
(e) Which gas turns blue cobalt chloride paper light pink?
Answer
(a) Chemical test to identify the gases are :
- Ammonia — When a glass rod dipped in conc. HCl is brought near the gas, dense white fumes of ammonium chloride are formed.
- Sulphur dioxide — Sulphur dioxide gas turns acidified potassium permanganate from pink to clear colourless and acidified potassium dichromate from orange to clear green.
- Hydrogen chloride — Forms a curdy white ppt. on passage through AgNO3 solution. The precipitate dissolves in excess of NH4OH.
- Chlorine — Pass the gas through silver nitrate solution, a white ppt. of silver chloride is formed.
- Carbon dioxide — When the gas is passed through lime water, it turns milky and white ppt. of calcium carbonate appears.
- Oxygen — Oxygen gas rekindles a glowing wooden splinter.
- Hydrogen — Hydrogen gas burns with a 'pop' sound in air.
(b) Ammonia is a basic gas. It turns red litmus blue.
(c) Sulphur dioxide, Hydrogen chloride, Chlorine, Carbon dioxide. It turns blue litmus red.
(d) Chlorine
(e) Water vapour
What is observed on performing the following :
| Hydrogen | Oxygen | Carbon dioxide | Chlorine | |
|---|---|---|---|---|
| Litmus test | ||||
| Applying burning splint to the gas | ||||
| Colour of gas | colourless | colourless | colourless | greenish yellow |
| Odour of gas |
Answer
| Hydrogen | Oxygen | Carbon dioxide | Chlorine | |
|---|---|---|---|---|
| Litmus test | Neutral to litmus | Neutral to litmus | blue litmus turns red | blue litmus turns red and finally bleaches |
| Applying burning splint to the gas | Pure hydrogen burns with a pale blue flame when a burning splinter is brought near it | rekindles a glowing splinter | burning splint is extinguished | burning splint is extinguished |
| Colour of gas | colourless | colourless | colourless | greenish yellow |
| Odour of gas | odourless | odourless | odourless | sharp pungent choking smell |
Give a chemical test to distinguish between the following gases.
(a) H2 and CO2
(b) H2 and O2
(c) CO2 and SO2
(d) HCl and H2S
(e) HCl and Cl2
(f) NH3 and HCl
(g) SO2 and Cl2
(h) NH3 and SO2
Answer
(a) H2 and CO2 — Hydrogen gas is neutral to litmus whereas carbon dioxide turns blue litmus red.
(b) H2 and O2 — Hydrogen gas burns with a pale blue flame when a burning splinter is brought near it whereas oxygen gas rekindles a glowing wooden splinter.
(c) CO2 and SO2 — Carbon dioxide gas has no effect on acidified potassium permanganate (KMnO4) and acidified potassium dichromate (K2Cr2O7) solution whereas sulphur dioxide gas turns acidified potassium permanganate from pink to clear colourless and acidified potassium dichromate from orange to clear green.
2KMnO4 + 2H2O + 5SO2 ⟶ K2SO4 + 2MnSO4 + 2H2SO4
K2Cr2O7 + H2SO4 + 3SO2 ⟶ K2SO4 + Cr2(SO4)3 + H2O
(d) HCl and H2S — Rod dipped in ammonia solution is brought near the mouth of the two gases. HCl gives dense white fumes of ammonium chloride and hence can be distinguished from hydrogen sulphide.
NH3(aq) + HCl ⟶ NH4Cl
(e) HCl and Cl2 — Chlorine (Cl2) turns moist starch iodide paper blue black whereas HCl does not.
Cl2 + 2KI ⟶ 2KCl + I2
Starch + I2 ⟶ Blue black colour
(f) NH3 and HCl — Ammonia turns Nessler's reagent from colourless to pale brown.
Hydrogen chloride shows no action with Nessler's reagent. It forms a curdy white ppt. on passage through AgNO3 solution.
AgNO3 [ag.] + HCl ⟶ AgCl ↓ [curdy white ppt.] + HNO3
The ppt. of AgCl is soluble in NH4OH but insoluble in dil. HNO3
(g) SO2 and Cl2 — On passing Sulphur dioxide gas through lime water, it turns lime water milky.
Ca(OH)2 + SO2 ⟶ CaSO3 ↓ [white ppt.] + H2O
Chlorine gas does not turn lime water milky. It turns moist starch iodide paper blue black.
Cl2 + 2KI ⟶ 2KCl + I2
Starch + I2 ⟶ Blue black colour
(h) NH3 and SO2 — Ammonia turns red litmus blue whereas sulphur dioxide turns wet blue litmus red and finally bleaches it, though the bleaching is temporary.
Name a gas that :
(a) turns moist starch iodide paper blue black
(b) turns moist red litmus blue
(c) does not affect acidified K2Cr2O7 paper but turns lime water milky.
(d) affects acidified K2Cr2O7 paper and also turns lime water milky.
Answer
(a) Chlorine
(b) Ammonia
(c) Carbon dioxide
(d) Sulphur dioxide
What do you observe when
(a) CO2 is passed through lime water first and then a little in excess?
(b) HCl is passed through silver nitrate solution?
(c) H2S is passed through lead nitrate solution?
(d) Cl2 is passed through potassium iodide (KI) solution?
(e) Cobalt chloride paper is introduced in water vapour?
Write the balanced equations for each of the above.
Answer
(a) When CO2 is passed through lime water first in small amounts it turns lime water milky. This is due to the formation of insoluble calcium carbonate.
Ca(OH)2 + CO2 ⟶ CaCO3 ↓ + H2O
When excess of the gas is passed through the solution, milkiness disappears. This is due to the formation of a soluble bicarbonate.
CaCO3 + CO2 + H2O ⟶ Ca(HCO3)2 [soluble]
(b) When HCl is passed through silver nitrate solution, a white ppt. of silver chloride is formed.
HCl + AgNO3 ⟶ AgCl ↓ + HNO3
(c) When H2S is passed through lead nitrate solution, black ppt. of PbS is formed.
Pb(NO3)2 [colourless] + H2S ⟶ PbS ↓ [black] + 2HNO3
(d) Cl2 turns potassium iodide (KI) solution blue black
Cl2 + 2KI ⟶ 2KCl + I2
(e) Water vapour turns blue cobalt chloride paper pink.
CoCl2 + 2H2O ⟶ CoCl2.2H2O
Name:
(a) Two carbonates that do not produce carbon dioxide on heating.
(b) Two nitrates that do not produce nitrogen dioxide on heating.
(c) A brown gas.
(d) A greenish yellow gas.
(e) A gas with rotten egg smell.
Answer
(a) Sodium carbonate [Na2CO3] and Potassium carbonate [K2CO3]
(b) Potassium nitrate [KNO3] and Sodium nitrate [NaNO3]
(c) Nitrogen dioxide [NO2]
(d) Chlorine [Cl2]
(e) Hydrogen sulphide [H2S]
Distinguish by heating the following in dry test tube.
(a) Zinc carbonate, copper carbonate and lead carbonate
(b) zinc nitrate and copper nitrate
(c) copper sulphate and copper carbonate
(d) ammonium chloride and iodine
Answer
(a) zinc carbonate, copper carbonate and lead carbonate :
On heating zinc carbonate, light amorphous white solid changes to pale yellow.
On heating copper carbonate, light green amorphous powder changes to black.
On heating lead carbonate, heavy white crystalline solid crumbles with a crackling sound.
(b) zinc nitrate and copper nitrate :
On heating zinc nitrate, white solid changes to yellow when hot and white when cold.
On heating copper nitrate, bluish green crystalline solid melts to form a bluish green mass and gives off steamy vapours which condense on cooler parts of the test tube.
On further heating, the bluish green mass changes to a black residue.
(c) copper sulphate and copper carbonate :
On heating copper sulphate, blue crystalline solid crumbles to form a white amorphous powder and gives off steamy vapours which condense on cooler parts of the test tube to form a colourless liquid (water).
On strong heating, a black solid is formed and a gas is evolved that turns moist blue litmus red and changes the colour of acidified K2Cr2O7 solution from orange to green suggesting that it is SO2.
On strongly heating copper carbonate, light green amorphous powder changes to black and a colourless odourless gas is evolved that extinguishes a burning wooden splinter and turns lime water milky but has no effect on acidified K2Cr2O7 solution suggesting that it is CO2.
(d) ammonium chloride and iodine :
On heating ammonium chloride, white crystalline solid sublimates to form a basic gas (NH3) and acidic gas (HCl). The dense white fumes are noticed that form a white mass on the cooler parts of the test tube.
On heating iodine, violet crystalline solid sublimates to form violet vapours. These vapours settle down on cooler parts of test tube to form violet crystals.
Match the following :
| Column A | Column B |
|---|---|
| (a) Pb(NO3)2 | (i) rotten egg smell |
| (b) CO2 | (ii) burns with a pop sound |
| (c) (NH4)2Cr2O7 | (iii) suffocating smell of sulphur |
| (d) HCl | (iv) lime water turns milky |
| (e) NO2 | (v) crackling sound |
| (f) O2 | (vi) residue swells up |
| (g) H2 | (vii) brown gas |
| (h) H2S | (viii) supports combustion |
| (i) SO2 | (ix) fumes with NH3 solution |
Answer
| Column A | Column B |
|---|---|
| (a) Pb(NO3)2 | (v) crackling sound |
| (b) CO2 | (iv) lime water turns milky |
| (c) (NH4)2Cr2O7 | (vi) residue swells up |
| (d) HCl | (ix) fumes with NH3 solution |
| (e) NO2 | (vii) brown gas |
| (f) O2 | (viii) supports combustion |
| (g) H2 | (ii) burns with a pop sound |
| (h) H2S | (i) rotten egg smell |
| (i) SO2 | (iii) suffocating smell of sulphur |
Distinguish by dilute sulphuric acid.
(a) Sodium sulphite and sodium carbonate.
(b) Copper and magnesium.
(c) Sodium sulphide and sodium sulphite.
Answer
(a) Sodium sulphite and sodium carbonate :
On adding dilute sulphuric acid to sodium sulphite and warming, a colourless gas with suffocating smell of burning sulphur is evolved. The evolved gas turns lime water milky and changes the colour of acidified K2Cr2O7 solution from orange to green suggesting that it is SO2.
Na2SO3 + H2SO4 (dil.) ⟶ Na2SO4 + H2O + SO2↑
On adding dilute sulphuric acid to sodium carbonate and warming, a colourless gas is evolved with brisk effervescence. The evolved gas extinguishes a burning wooden splinter and turns lime water milky but has no effect on acidified K2Cr2O7 solution suggesting that it is CO2.
Na2CO3 + H2SO4 (dil.) ⟶ Na2SO4 + H2O + CO2↑
(b) Copper and magnesium :
Magnesium on reaction with dil. sulphuric acid produces a colourless, odourless gas with brisk effervescence. The gas evolved is hydrogen as it burns with a pale blue flame producing a pop sound.
Copper does not react with dil. sulphuric acid liberating hydrogen as it is lower in metal reactivity series than hydrogen.
(c) Sodium sulphide and sodium sulphite :
When dil. sulphuric acid is added to sodium sulphide and heated, hydrogen sulphide gas is evolved which has the smell of rotten eggs.
Na2S + H2SO4 ⟶ Na2SO4 + H2S ↑
On adding dilute sulphuric acid to sodium sulphite and warming, a colourless gas with suffocating smell of burning sulphur is evolved. The evolved gas turns lime water milky and changes the colour of acidified K2Cr2O7 solution from orange to green suggesting that it is SO2.
Na2SO3 + H2SO4 (dil.) ⟶ Na2SO4 + H2O + SO2↑
Write your observation and a balanced equation in the case of the following substances being heated.
(a) Ammonium dichromate
(b) Copper nitrate
(c) Copper carbonate
(d) Zinc carbonate
(e) Ammonium chloride
Answer
(a) Ammonium dichromate — Orange red crystalline solid, on heating, swells up and decomposes violently with flashes of light leaving greenish residue. It also gives off steamy fumes, which condense on the cooler parts of the test tube to form tiny droplets of water.
(b) Copper nitrate — Bluish green crystalline solid, on heating, melts to form a bluish green mass and gives off steamy vapours that condense on the cooler parts of test tube.
On further heating, the bluish green mass changes to a black residue of copper (II) oxide and brown coloured nitrogen dioxide gas is evolved along with oxygen gas.
(c) Copper carbonate — Light green amorphous powder changes to black. Carbon dioxide gas is given off which turns lime water milky.
(d) Zinc carbonate — On strong heating, light amorphous white solid changes to pale yellow. Carbon dioxide gas is given off which turns lime water milky.
The residue, on cooling, changes to a white colour. i.e., residue is yellow when hot and white when cold.
(e) Ammonium chloride — On heating ammonium chloride, white crystalline solid sublimates to form a basic gas (NH3) and acidic gas (HCl). The dense white fumes are noticed that form a white mass on the cooler parts of the test tube. No residue is left behind.
State the original colour of the following substances and colour of residue obtained after heating.
(a) ammonium dichromate
(b) copper carbonate
(c) lead nitrate
(d) zinc carbonate
Answer
| S. No. | Salt | Original colour | Residue after heating |
|---|---|---|---|
| (a) | ammonium dichromate | Orange solid | Green solid |
| (b) | copper carbonate | Green solid | Black solid |
| (c) | lead nitrate | White solid | Yellow solid |
| (d) | zinc carbonate | White solid | Yellow when hot, white when cold |
Carbon dioxide and sulphur dioxide gas can be distinguished by using :
moist blue litmus paper
lime water
acidified potassium dichromate paper
none of the above
Answer
acidified potassium dichromate paper
Reason — There is no effect of CO2 gas on potassium dichromate whereas SO2 turns acidified potassium dichromate from orange to clear green.
K2Cr2O7 + H2SO4 + 3SO2 ⟶ K2SO4 + Cr2(SO4)3 + H2O
Assertion (A): Chlorine turns moist blue litmus red and finally decolourises it.
Reason (R): Chlorine is acidic in nature and an oxidising agent.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true and R is the correct explanation of A.
Explanation — Chlorine reacts with water to form hydrochloric acid (HCl) and hypochlorous acid (HClO).
Cl2 + H2O HCl + HClO
These acids make the solution acidic, turning moist blue litmus red and due to the oxidising nature of HClO, the dye in the litmus paper is bleached, leading to discolourisation. Hence, both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A).
Assertion (A): Glass rod dipped in NH4OH gives dense white fumes with HCl gas.
Reason (R): HCI gas is acidic in nature.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
Both A and R are true but R is not the correct explanation of A.
Explanation — When a glass rod is dipped in ammonia solution and brought near the gas, dence white fumes of ammonium chloride are formed. Hence, the assertion (A) is true.
NH3 (aq) + HCl ⟶ NH4Cl
Hydrochloric acid (HCl) is strongly acidic in nature. Hence, the reason (R) is true.
Reason (R) doesn't explain assertion because the formation of white fumes is specifically due to the reaction between NH3 and HCl forming NH4Cl, not merely because HCl is acidic. Hence, the reason (R) is not the correct explanation of assertion (A).
Assertion (A): Zinc nitrate on heating produces a colourless gas, brown gas and yellow residue which fuses with the glass.
Reason (R): Zinc nitrate decomposes to give ZnO, NO2 and O2.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is false but R is true.
Explanation — On heating zinc nitrate melts and forms a white sticky mass. The colourless gas, brown gas and yellow residue is formed only when the zinc nitrate is heated strongly. Hence, the assertion (A) is false.
Zinc nitrate on heating strongly decomposes to give ZnO, NO2 and O2. Hence, the reason (R) is true.
Assertion (A): Sodium chloride and potassium chloride can be distinguished by flame test.
Reason (R): Sodium salt produces a red flame and potassium salt produces a lilac flame.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true but R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
Answer
A is true but R is false.
Explanation — Sodium chloride and potassium chloride can be distinguished using flame text. Sodium salt produces golden yellow flame and potassium salt produces lilac flame. Hence, the assertion (A) is true.
Sodium salt produces golden yellow flame rather than red flame and potassium salt produces lilac flame. Hence, the reason (R) is false.
Match the gases in column I to the identification of the gases mentioned in column II
| Column I | Column II |
|---|---|
| (a) Hydrogen sulphide | A. Turns acidified potassium dichromate solution green |
| (b) Nitric oxide | B. Turns lime water milky |
| (c) Carbon dioxide | C. Turns reddish brown when it reacts with oxygen |
| (d) Sulphur dioxide | D. Turns moist lead acetate paper silvery black |
Answer
| Column I | Column II |
|---|---|
| (a) Hydrogen sulphide | D. Turns moist lead acetate paper silvery black |
| (b) Nitric oxide | C. Turns reddish brown when it reacts with oxygen |
| (c) Carbon dioxide | B. Turns lime water milky |
| (d) Sulphur dioxide | A. Turns acidified potassium dichromate solution green |
Match column A with column B
| Column A | Colum B |
|---|---|
| (a) Blue salt changes to white and then black | (i) Ammonium dichromate |
| (b) Orange coloured compound changes to green | (ii) Iodine |
| (c) Red compound changes to brown and then yellow | (iii) Zinc Nitrate |
| (d) White to yellow when hot and white when cold | (iv) Copper Sulphate |
| (e) Violet solid changes to violet vapours | (v) Red Lead |
Answer
| Column A | Colum B |
|---|---|
| (a) Blue salt changes to white and then black | (iv) Copper Sulphate |
| (b) Orange coloured compound changes to green | (i) Ammonium dichromate |
| (c) Red compound changes to brown and then yellow | (v) Red Lead |
| (d) White to yellow when hot and white when cold | (iii) Zinc Nitrate |
| (e) Violet solid changes to violet vapours | (ii) Iodine |
Identify the following substances:
(a) An alkaline gas A which gives dense white fumes with hydrogen chloride.
(b) Gas B that has an offensive smell as of rotten eggs.
(c) Gas C that is colourless and can be use as a bleaching agent.
(d) A brown gas D with irritating smell.
Answer
(a) A → Ammonia
(b) B → Hydrogen sulphide
(c) C → Sulphur dioxide
(d) D → Nitrogen dioxide
Name the gases which :
(a) extinguishes burning wooden splinter.
(b) turns moist red litmus blue.
(c) do no effect on moist litmus.
(d) affect the acidified K2Cr2O7 paper and also turns lime water dirty milky.
Answer
(a) NH3, HCl, SO2, H2S, CO2, NO2, Cl2.
(b) Ammonia [NH3]
(c) Water vapour, hydrogen, oxygen
(d) Sulphur dioxide [SO2]
What do you observe when dilute sulphuric acid is added to the following:
(a) Sodium Sulphide
(b) Sodium Carbonate
(c) Zinc granules
Answer
(a) When dil. sulphuric acid is added to sodium sulphide and heated, hydrogen sulphide gas is evolved which has the smell of rotten eggs and turns lead acetate paper black.
Na2S + H2SO4 ⟶ Na2SO4 + H2S
(b) When dilute sulphuric acid is added to sodium carbonate a colourless gas [CO2] is evolved with brisk effervescence that turns lime water milky and has no effect on acidified potassium dichromate paper.
Na2CO3 + H2SO4 ⟶ Na2SO4 + H2O + CO2 (g)
(c) When dilute sulphuric acid is added to granulated zinc, hydrogen gas is evolved with an effervescence.
Zn + H2SO4 (dil) ⟶ ZnSO4 + H2 (g)
How is the flame test performed?
Answer
The procedure to detect the colour imparted by flame test is as follows:
A thin platinum wire is first thoroughly cleaned by dipping it in conc. hydrochloric acid. It is then heated in the non-luminous flame of the burner. The process is repeated. When the wire imparts no colour to the flame, it is ready for use.
Now, wire is first dipped in conc. hydrochloric acid and then in the salt to be tested. It is then introduced into the non-luminous part of the flame and the colour imparted to the flame is observed.
How will you distinguish between sodium chloride, potassium chloride and calcium chloride?
Answer
We can distinguish between the three salts [sodium chloride, potassium chloride and calcium chloride] with the help of flame test as described below:
Method
- Thin platinum wire is thoroughly cleaned and then heated in a non-luminous flame of a burner.
- When the wire imparts no colour it is dipped in conc. HCl and then into the substance to be identified.
- The wire is then reintroduced into the non-luminous flame and the colour imparted is noted.
Observation —
- The golden yellow flame confirms the presence of sodium [Na1+] ion.
- Lilac colour of flame confirms the presence of Potassium [K1+]
- Brick red colour of flame, confirms the presence of Calcium [Ca2+] ion.
How will you distinguish between soft water and hard water
Answer
Differentiating hard water from soft water —
- Two unknown samples 'X' and 'Y' containing hard water and soft water are taken separately in a trough or beaker.
- Ordinary soap is rubbed by the hands inside each sample.
Observation —
- One sample of water 'X' lathers with soap
- The sample of water 'Y' does not lather with soap.
Result —
- The sample 'X' which lathers with soap is soft water.
- The sample 'Y' which does not lather with soap is hard water.
How will you distinguish between temporary hard water and permanent hard water
Answer
Distinguish between temporary hard water and permanent hard water —
- Two unknown samples 'A' and 'B' containing temporary and permanent hard water are taken separately in a trough or beaker.
- The water is boiled slowly, gases allowed to escape out and then the water is filtered.
- Ordinary soap is rubbed by the hands inside each filtered sample.
Observation —
- One sample of water 'A' lathers with soap.
- The sample of water 'B' does not lather with soap.
Result —
- The boiled and filtered sample 'A' which lathers is temporary hard water whose hardness is removed by boiling.
- Sample 'B' is permanent hard water whose hardness cannot be removed by boiling.
(a) What do you understand by :
(i) temporary hardness
(ii) soft water
(iii) permanent hardness
(b) How are temporary and permanent hardness removed ?
Answer
(a) (i) Temporary hardness — Hardness of water due to the presence of calcium and magnesium bicarbonates — Ca(HCO3)2, Mg(HCO3)2 — that can be removed by boiling and filtration is called temporary hardness.
(ii) Soft water — Water that does not contain dissolved calcium and magnesium salts is called soft water. This water forms lather with ordinary soap.
(iii) Permanent hardness — Hardness of water due to the presence of calcium and magnesium chlorides and sulphates — CaCl2, MgCl2, CaSO4, MgSO4 — that cannot be removed by boiling is called permanent hardness.
(b) Temporary hardness can be removed by boiling followed by filtration and permanent hardness can be removed by addition of washing soda or caustic soda.
(a) What are soaps and detergents?
(b) Why do they differ in their actions ?
(c) Explain their cleansing actions.
Answer
(a) Soap — It is the sodium or potassium salt of an organic fatty acid. It reacts with hard water forming scum, which is why ordinary soap is wasted.
CaSO4 + 2NaSt (soap) ⟶ CaSt2 (scum) + Na2SO4
Detergents — They are the sodium salts of alkyl sulphonic acids. Detergents contain a sulphonic acid group (-SO3H) instead of a carboxylic group (-COOH).
(b) Soaps reacts with hard water forming scum, which is why ordinary soap is wasted.
Detergents can lather even with hard water. Due to the solubility of their calcium and magnesium salts in water, they do not form scum.
(c) Soap or detergent molecules form clusters called micelles when dissolved in water. The molecules arrange themselves with their tails inward and their heads outward. During the cleansing process, the hydrocarbon tail of the detergent attaches to oil and dirt. When water is stirred, the oil and dirt separate into smaller fragments. This allows other tails to attach to the fragmented oil and dirt. The detergent molecules surround these small oil and dirt globules. The negatively charged heads in the water prevent the globules from recombining into larger aggregates. As a result, when clothes are rinsed with water, the oil and dirt are effectively removed.

Compare the effect of soap and detergent on hard water.
Answer
Soaps react with hard water forming scum which is why ordinary soap is wasted whereas detergents can lather even with hard water. Due to the solubility of their calcium and magnesium salts in water, they do not form scum.
Copy and complete the following table that refers to the action of heat on some carbonates:
| Carbonate | Colour of residue on cooling |
|---|---|
| Zinc carbonate | |
| Lead carbonate | |
| Copper carbonate |
Answer
| Carbonate | Colour of residue on cooling |
|---|---|
| Zinc carbonate | Yellow when hot, white when cold |
| Lead carbonate | Yellow |
| Copper carbonate | Black |
Complete the following table and write your observations.
| Hydrogen sulphide | Ammonia | Sulphur dioxide | Hydrogen chloride | |
|---|---|---|---|---|
| Shake the gas with red litmus solution | ||||
| Shake the gas with blue litmus solution | ||||
| Apply a burning splint to the gas |
Answer
| Hydrogen sulphide | Ammonia | Sulphur dioxide | Hydrogen chloride | |
|---|---|---|---|---|
| Shake the gas with red litmus solution | No change | Red litmus becomes blue | No change | No change |
| Shake the gas with blue litmus solution | Blue litmus becomes red | No change | Blue litmus becomes red | Blue litmus becomes red |
| Apply a burning splint to the gas | Burning splint is extinguished | Burning splint is extinguished | Burning splint is extinguished | Burning splint is extinguished |
A series of experiments were performed as shown below to prepare certain gases in the laboratory.
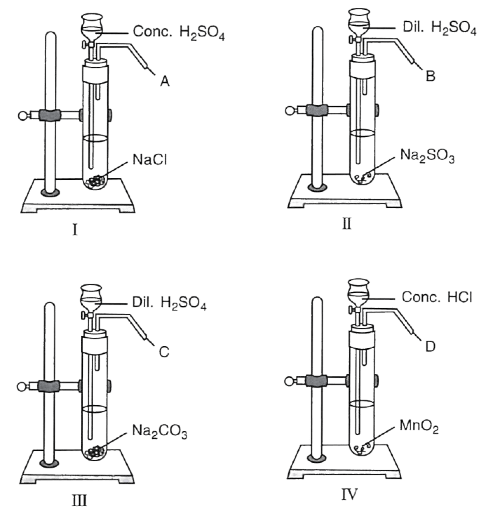
(i) Name the gas A. What do you observe when gas A is passed through AgNO3 solution ?
(ii) Name the gas B. What test will you perform to identify gas B.
(iii) What is the similarity between gas B and gas C ?
(iv) Give a test to distinguish between gases B and C.
(v) In experiment IV, gas D is :
P — HCl
Q — Cl2
R — O2
- Only P
- Only Q
- Only R
- Both P and Q
(vi) Write the equations for when gas B and gas C are separately passed in lime water first in little quantities and then in excess.
(vii) When a rod dipped in ammonium hydroxide solution is brought near gas ............... dense white fumes are seen.
P — Gas A
Q — Gas B
R — Gas D
- Only P
- Only Q
- Only R
- Both P and R
Answer
(i) Gas A is HCl, When the HCl is passed through silver nitrate solution, a white precipitate of silver chloride is formed.
AgNO3 (aq) + HCl ⟶ AgCl ↓ + HNO3
(ii) Gas B is SO2 , it decolorises purplish pink acidified potassium permanganate solution.
(iii) Gas B is SO2 and Gas C is CO2, they are colourless, acidic gases which can turn lime water milky (turbid).
(iv) Gas B (SO2) decolorises purplish pink acidified potassium permanganate solution and it can change orange/yellow solution of acidified potassium dichromate green. Gas C (CO2) has no effect on acidified potassium permanganate solution and acidified potassium dichromate solution.
(v) Only Q,
MnO2 + 4HCl ⟶ MnCl2 + H2O + Cl2
(vi)
CO2 in limited Quantity:
CO2 passed in excess:
SO2 in limited Quantity:
SO2 passed in excess:
(vii) Only P
When a glass rod dipped in ammonia solution and brought near the gas A i.e., HCl, dense white fumes of ammonium chloride are formed.
NH3 (aq) + HCl ⟶ NH4Cl