Ravi was asked to identify the cation present in the salt solution. He added one of the reagents given below and got a reddish-brown precipitate. The reagent that he used is:
- Silver nitrate solution
- Barium chloride solution
- Ammonium hydroxide
- Calcium chloride solution
Answer
Ammonium hydroxide
Reason — When iron (III) salts, having Fe3+ ions, are treated with ammonium hydroxide they give reddish brown precipitate, which is not soluble in excess ammonium hydroxide.
Which metal does not react with HCl to form a colourless, odourless gas which burns with a pop sound?
- Ca
- Mg
- Cu
- Zn
Answer
Cu
Reason — Hydrochloric acid reacts with metals like Ca, Mg and Zn, which are above hydrogen in the activity series forming metallic chlorides and evolving hydrogen, it is a colourless, odourless gas which burns with a pop sound.
Whereas, Cu is less reactive than hydrogen and present below hydrogen in the activity series, thus it cannot displace hydrogen from HCl.
Prateek added warm water to magnesium nitride, and a colourless gas evolved, which, when tested with phenolphthalein, turned it pink. The gas evolved is:
- Carbon dioxide
- Ammonia
- Nitrogen
- Hydrogen chloride
Answer
Ammonia
Reason — On adding warm water to magnesium nitride, colourless, basic ammonia gas is evolved. When tested with phenolphthalein, a basic substance will turn the indicator pink, confirming the presence of a base.
Mg3N2 + 6H2O ⟶ 3Mg(OH)2 + 2NH3 [g]
Which of the following statements about ethane is false?
- It is a saturated hydrocarbon.
- It undergoes a substitution reaction.
- It is a gas at ordinary temperatures.
- It has a triple bond between the carbon atoms.
Answer
It has a triple bond between the carbon atoms.
Reason — Ethane is a alkane(CnH2n+2) in which all the four valencies of carbon are fully satisfied by single bonds.
Thermite mixture is used to weld the broken ends of the iron girders. This mixture consists of ferric oxide and aluminium powder, which, when heated, produces molten iron. In this reaction, the aluminium powder acts as a/an ............... agent.
- oxidising
- reducing
- dehydrating
- corroding
Answer
reducing
Reason — When a mixture of aluminium powder and ferric oxide is ignited, the ferric oxide is reduced to metal. This process is called aluminothermy. Here, Aluminium acts as a powerful reducing agent.
The below diagram represents the electrolysis of acidulated water. The reaction occurring at the anode is:
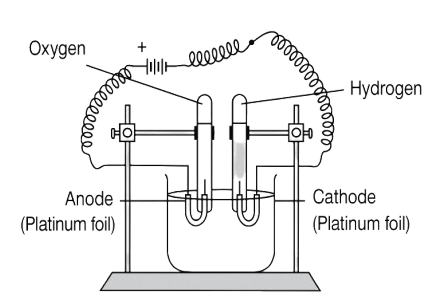
- H2SO4 ⟶ 2H+ + SO42-
- H2O ⟶ H+ + OH-
- H+ + e- ⟶ H, 2[H] + 2[H] ⟶ H2
- OH- - e- → OH, [4OH] ⟶ 2H2O + O2
Answer
OH- - e- → OH, [4OH] ⟶ 2H2O + O2
Reason — OH- migrates to the anode. OH- being lower in the electro-chemical series is discharged preferentially. OH- loses one electron to the anode and becomes neutral OH.
OH- - e- → OH
The combination of OH forms water with the liberation of oxygen, which is given off at the anode.
[4OH] ⟶ 2H2O + O2
| Group number | 1A 1 | IIA 2 | IIIA 13 | IVA 14 | VA 15 | VIA 16 | VIIA 17 | VIIIA 18 |
|---|---|---|---|---|---|---|---|---|
| Li | D | O | J | Ne | ||||
| A | Mg | E | Si | H | K | |||
| B | C | F | G | L |
With reference to the portion of the periodic table given above, identify the element having the largest atomic size:
- Li
- B
- K
- L
Answer
B
Reason — B has the largest atomic size, because,
Down the group — The size of an atom increases as one proceeds from top to bottom. Since B is present at third position of first group, it has larger size due to the successive addition of shells.
Across the period — The size of the atom decreases from left to right. Since B is the first element of third period, it has largest atomic size.
The picture given below shows an apparatus that a teacher used for demonstrating the properties of ionic substances. The teacher heats a sample of lead bromide in a crucible which contains two electrodes which are part of the circuit shown. The bulb does not light up. What is the best explanation for this?
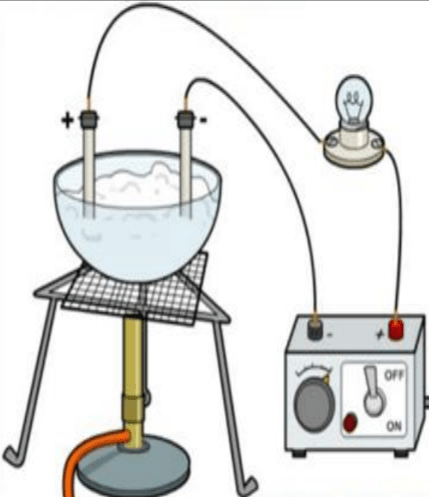
- The circuit is complete.
- Molten lead bromide does not conduct electricity.
- The sample of lead bromide was not heated up to the melting point by the teacher.
- The DC power supply was set up correctly.
Answer
The sample of lead bromide was not heated up to the melting point by the teacher.
Reason — To conduct electricity the crucible containing lead bromide should be heated above 380°C, the melting point of Lead bromide. Solid lead bromide is a non-conductor of electricity, since its ions are not free. The ions become free when lead bromide is in the fused or molten state. Thus, in the above experiment the sample of lead bromide was not heated up to the melting point by the teacher. So, the bulb does not light up.
Element Y is in Group IIA of the Periodic Table. Y reacts with element Q to form an ionic compound. Which equation shows the process that takes place when Y forms ions?
- Y + 2e- ⟶ Y2+
- Y - 2e- ⟶ Y2-
- Y + 2e- ⟶ Y2-
- Y - 2e- ⟶ Y2+
Answer
Y - 2e- ⟶ Y2+
Reason — Group IIA elements are alkaline earth metals with 2 electrons in their outermost shell. To achieve a stable noble gas configuration, they prefer to lose these 2 electrons rather than gain more forming a positive ion. Hence, the equation will be
Y - 2e- ⟶ Y2+
The diagram below shows a circuit used to electrolyse aqueous sodium argento cyanide. Which arrow indicates the movement of the silver ions in the electrolyte and of the electrons in the external circuit?
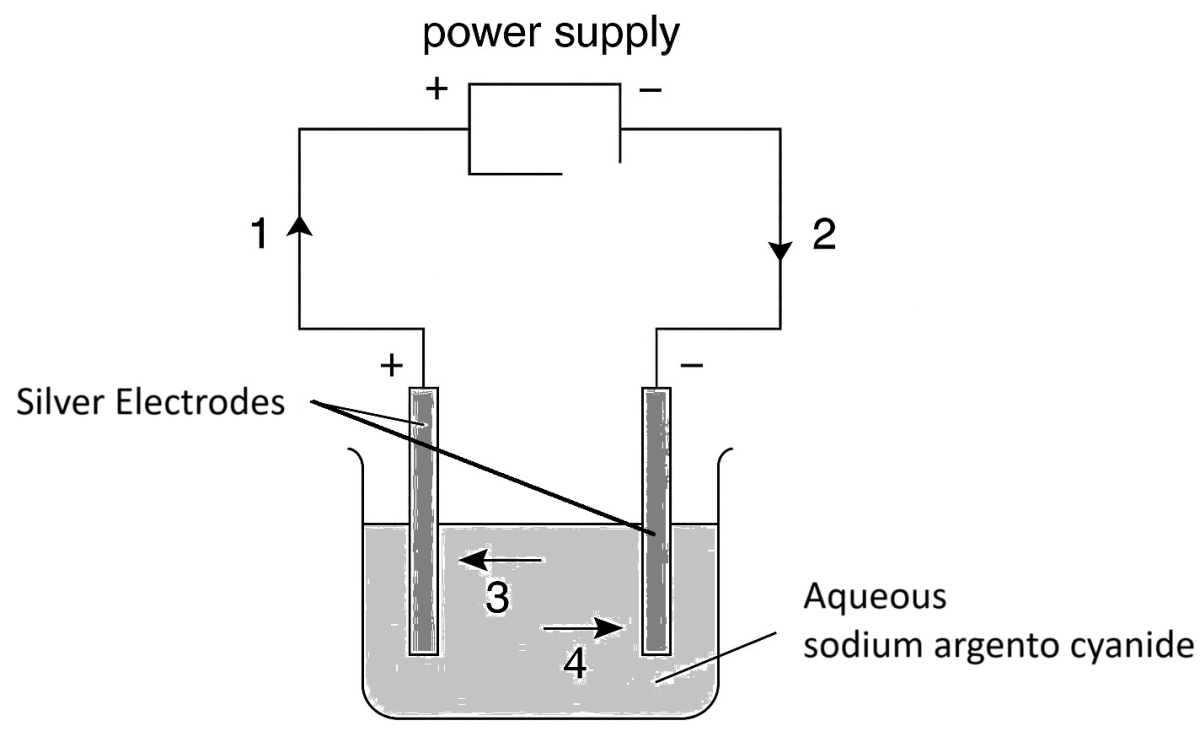
| Silver ions | Electrons | |
|---|---|---|
| 1. | 3 | 1 |
| 2. | 3 | 2 |
| 3. | 2 | 4 |
| 4. | 4 | 1 |
Answer
4,1
Reason — The silver ions are released and passed into solution from the active anode electrode, and they migrate towards the cathode. Hence, the direction of movement of silver ions will be 4.
The direction of current in the external circuit will be from positive terminal of the power supply to the negative terminal. The movement of electrons in the external circuit will in the opposite direction of current. Hence, the direction of movement of electrons is 1.
The relative atomic mass of nitrogen is 14, and that of hydrogen is 1. This means that (i) ............... of nitrogen has the same mass as (ii) ............... of hydrogen.
| (i) | (ii) | |
|---|---|---|
| 1. | An atom | 28 molecules |
| 2. | An atom | 7 molecules |
| 3. | A molecule | 14 atoms |
| 4. | A molecule | 7 atoms |
Which words correctly complete the gaps?
Answer
An atom / 7 molecules
Reason — Atomic mass of Nitrogen is 14, atomic mass of hydrogen is 1.
7 molecules of hydrogen = 7 x H2 = 7 x 2 (H) = 7 x 2 = 14.
Hence, atomic mass of one atom of nitrogen is same as 7 molecules of Hydrogen.
A student reacts copper turnings with cold dilute nitric acid in a test tube. He tests the gas given off with moist red and blue litmus paper. What is the name of the gas that evolved and what is the final colour of the litmus paper?
| Gas | Final colour of the litmus paper | |
|---|---|---|
| 1. | NO | No change in blue and red litmus paper |
| 2. | NO2 | Blue litmus turns red and no change in red litmus |
| 3. | N2 | No change in blue and red litmus paper |
| 4. | N2O | No change in blue and red litmus paper |
Answer
NO, No change in blue and red litmus paper
Reason — Nitric oxide is evolved when cold and dilute HNO3 is added to copper. It is neither acidic nor basic so, it does not change the colour of red or blue litmus paper
3Cu + 8HNO3 ⟶ 3Cu(NO3)2 + 4H2O + 2NO
Which element forms a stable ion with the same electronic configuration as argon?
- Magnesium
- Fluorine
- Chlorine
- Sodium
Answer
Chlorine
Reason — The electronic configuration of chlorine is 2, 8, 7. It has an electronic configuration with one electron less than that of the nearest noble gas, argon (2, 8, 8).
By acquiring an electronic configuration of argon, it will have one electron more than the number of protons in its nucleus, Hence, it will gain charge of -1.
The diagram below represents the molecule of an organic compound. What is the name of this compound?
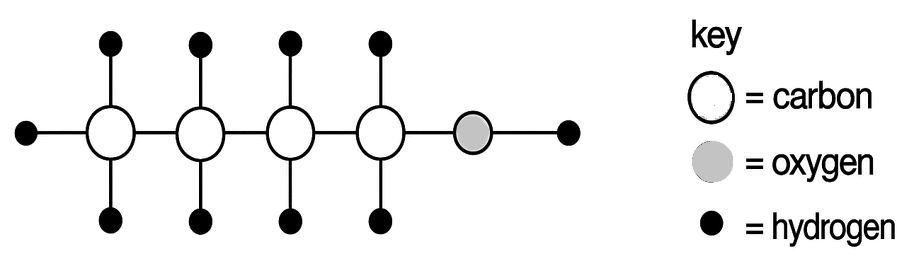
- Pentanol
- Butanol
- Butanoic acid
- Pentanoic acid
Answer
Butanol
Reason — The diagram shows presence of four carbon atoms linked with hydrogen by single bond and a -OH at the end as a functional group. So, "Buta" for four carbons and saturated hydrocarbon and -ol for the alocohol group.
When a compound was electrolysed using inert electrodes, the gas released at the anode made a glowing splinter rekindle. The electrolyte that will not produce such gas observation at the anode is:
- diluted solution of NaCl.
- concentrated solution of NaCl.
- diluted solution of copper sulphate.
- acidified water.
Answer
concentrated solution of NaCl.
Reason — A gas released at the anode that caused a glowing splinter to rekindle is oxygen gas (O2). Concentrated solution of NaCl doesn't produce oxygen gas but it produces Chlorine which does not rekindle a glowing splinter.
Whereas, dilute HCl, dilute copper sulphate and acidified water produce oxygen gas when they are electrolysed using inert electrodes.
Which of the following chains of hydrocarbons undergoes two steps of reactions to become saturated?
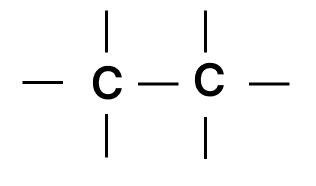
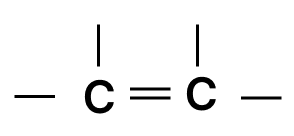
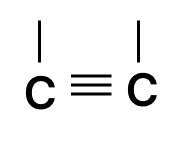
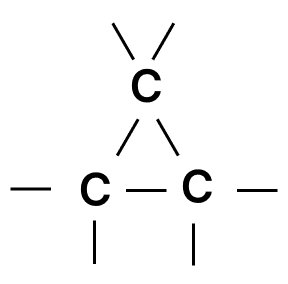
Answer
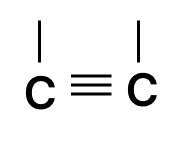
Reason — Triple bonds are converted to single bonds in two steps: first, the triple bond becomes a double bond, then the double bond becomes a single bond. So, the chain with the triple bond needs two reactions to become fully saturated.
Given below are four different illustrations of preparing hydrochloric acid drawn by students. Which of these is the correct?
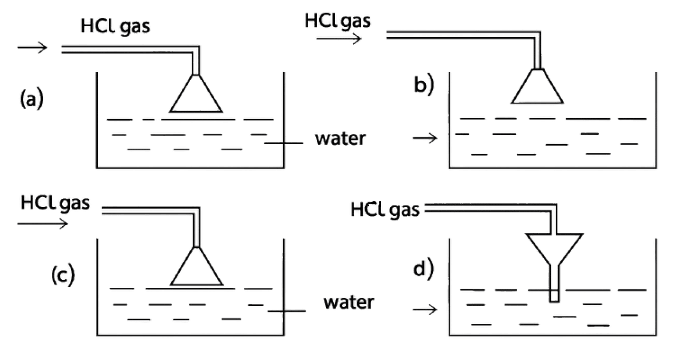
Answer
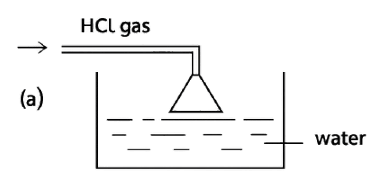
Reason — The inverted funnel is placed so that its rim just touches the water surface in the beaker. This setup is important for two main reasons:
- To prevent back-suction of water:
If the funnel mouth were dipped too deep into the water, a sudden cooling or stopping of gas flow could create a partial vacuum and suck water back into the gas supply tube. Keeping the rim just touching the water avoids this backward flow of water. - To increase the surface area for absorption:
The wide mouth of the funnel spreads the hydrogen chloride gas over a larger area of water. This helps the gas dissolve faster and more efficiently, since hydrochloric acid is prepared by dissolving hydrogen chloride gas in water.
When two organic compounds A and B react together in the presence of conc. H2SO4, a fruity smell evolved from one of the products. If A has the functional group [-O-H], which of the following stands for the functional group of B?
![When two organic compounds A and B react together in the presence of conc. H2 SO4, a fruity smell evolved from one of the products. If A has the functional group [-O-H], which of the following stands for the functional group of B? Chemistry Competency Focused Practice Questions Class 10 Solutions.](https://cdn1.knowledgeboat.com/img/cp10/q18-a-mcq-concise-chem-competency-practice-2026-solutions-class-10-icse-239x162.png)
![When two organic compounds A and B react together in the presence of conc. H2 SO4, a fruity smell evolved from one of the products. If A has the functional group [-O-H], which of the following stands for the functional group of B? Chemistry Competency Focused Practice Questions Class 10 Solutions.](https://cdn1.knowledgeboat.com/img/cp10/q18-b-mcq-concise-chem-competency-practice-2026-solutions-class-10-icse-252x123.png)
![When two organic compounds A and B react together in the presence of conc. H2 SO4, a fruity smell evolved from one of the products. If A has the functional group [-O-H], which of the following stands for the functional group of B? Chemistry Competency Focused Practice Questions Class 10 Solutions.](https://cdn1.knowledgeboat.com/img/cp10/q18-c-mcq-concise-chem-competency-practice-2026-solutions-class-10-icse-319x128.png)
![When two organic compounds A and B react together in the presence of conc. H2 SO4, a fruity smell evolved from one of the products. If A has the functional group [-O-H], which of the following stands for the functional group of B? Chemistry Competency Focused Practice Questions Class 10 Solutions.](https://cdn1.knowledgeboat.com/img/cp10/q18-d-mcq-concise-chem-competency-practice-2026-solutions-class-10-icse-181x97.png)
Answer
![When two organic compounds A and B react together in the presence of conc. H2 SO4, a fruity smell evolved from one of the products. If A has the functional group [-O-H], which of the following stands for the functional group of B? Chemistry Competency Focused Practice Questions Class 10 Solutions.](https://cdn1.knowledgeboat.com/img/cp10/q18-c-mcq-concise-chem-competency-practice-2026-solutions-class-10-icse-319x128.png)
Reason — When an alcohol (–OH group) reacts with a carboxylic acid (–COOH group) in the presence of concentrated sulphuric acid, an ester is formed. Esters have a pleasant fruity smell. So, since A is an alcohol, B must be a carboxylic acid.
Example:
Given below are four covalent compounds.
(A) H2O (B) CCl4 (C) Cl2 (D) NH3
Which of the following represents the correct order when they are arranged in their increasing number of covalent bonds?
- B < D < A < C
- A < C < D < B
- C < D < A < B
- C < A < D < B
Answer
C < A < D < B
Reason
| Molecule | No. of covalent bonds | |
|---|---|---|
| A | H2O | 2 |
| B | CCl4 | 4 |
| C | Cl2 | 1 |
| D | NH3 | 3 |
So, the right order of increasing number of covalent bonds is C < A < D < B
The electrolytic cell used for the electrolysis of molten lead bromide is made of Silica. Which of the following properties of silica that is the reason for it not having much significance in the process of electrolysis?
- Hard and strong
- Non-conductor of electricity
- Non-reactive
- Withstands high temperature
Answer
Hard and strong
Reason — Silica is non-reactive, can withstand high temperature and is almost a non-conductor of electricity. Hence, the elctrolytic cell is made of silica. However, hard and strong nature of silica does not have much significance in the process of electrolysis.
A distinctive reaction that takes place when ethanol is treated with acetic acid in the presence of concentrated sulphuric acid to give a fruity smell.
P: The reaction is called esterification.
Q: The reaction is called hydration.
- Only P
- Only Q
- Both P and Q
- Both P and Q are wrong
Answer
Only P
Reason — A distinctive reaction that takes place when ethanol is treated with acetic acid in the presence of concentrated sulphuric acid to give a ester with fruity smell is esterification.
The pH of the soil is tested, and for the better growth of crops, slightly alkaline soil is required. Which ion in the fertiliser will increase the alkalinity of the soil?
- Hydronium ion
- Hydroxyl ion
- Hydrogen ion
- Both hydroxyl and hydrogen
Answer
Hydroxyl ion
Reason — To increase the alkalinity (i.e., raise the pH) of the soil, we need to reduce acidity and introduce basicity. Basic (alkaline) substances increase OH- (hydroxyl ions). Hydroxyl ions neutralize H+ in the soil and raise the pH, making it more alkaline.
Ramu makes a detailed study on the values of electronegativity and the formation of compounds. Accordingly, he draws the following conclusion:
The larger the electronegativity (EN) difference between the combining atoms, the more ionic bonds will form.
If the EN difference is negligible, covalent bonds will form. So, which of the following values refers to covalent bonds?
P: 3.0 and 3.0
Q: 0.9 and 3.0
- Only P
- Only Q
- Both P and Q
- Neither P nor Q
Answer
Only P
Reason — The condition for the formation of a covalent bond is the electronegative difference between the combining atoms should either be zero or negligible.
P: 3.0 and 3.0, the electronegative difference is zero, leading to covalent bond formation.
Q: 0.9 and 3.0, the electronegative difference is 2.1, NO covalent bond formation.
10g of magnesium carbonate reacts completely with excess dilute hydrochloric acid. What volume of carbon dioxide is formed at room temperature and pressure? [Mg=24, C=12, O=16] The equation for the reaction is:
MgCO3 + 2HCl ⟶ MgCl2 + H2O + CO2
- 2.8 dm3
- 2.6 dm3
- 2.2 dm3
- 2.4 dm3
Answer
2.6 dm3
Reason —
1 mole of MgCO3 gives 1 mole of CO2
Molar Mass of MgCO3
MgCO3 = 1 x 24 + 1 X 12 + 3 X 16 = 84 g/mol
∴ No. of moles of MgCO3 in 10g
Moles of MgCO3 = = 0.119 mol
From the reaction, 1 mole of MgCO2 gives 1 mole of CO2,
∴ Moles of CO2 = 0.119mol
Volume of CO2 at RTP,
= 0.119 X 22.4 = 2.6 dm3
∴ volume of carbon dioxide at RTP is 2.6 dm3
The diagram shown is a wrong attempt to electroplate a pan with copper:
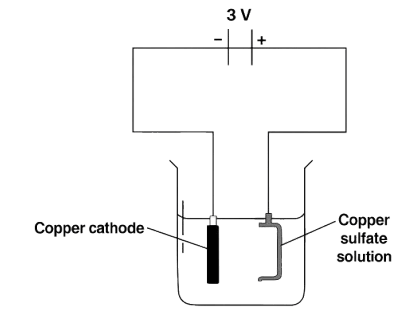
Which of the following could have been done to copper plate a pan?
- To change DC to AC.
- To change the electrolyte from copper sulphate to cobalt sulphate.
- Connect the pan to the negative electrode.
- To induce a higher current.
Answer
Connect the pan to the negative electrode.
Reason — The article to be electroplated is always placed at the cathode, during electrolytic reaction, the metal is always deposited at the cathode by gain of electrons.
During the extraction of aluminium by Hall Heroult’s process, the carbon rods are replaced continuously. This is because:
- It minimises heat loss by radiation.
- It enhances the mobility of ions.
- The carbon anode is consumed.
- It lowers the fusion point.
Answer
The carbon anode is consumed.
Reason — During the extraction of aluminium by Hall Heroult’s process, the carbon rod are replaced time to time, as it get oxidised by the oxygen evolved at the anode and get consumed.
Which of the following observations correctly shows the action of indicator on sodium hydroxide solution?
| Indicator | methyl orange | phenolphthalein | |
|---|---|---|---|
| 1. | P | orange to yellow | remains colourless |
| 2. | Q | orange to pink | remains colourless |
| 3. | R | orange to yellow | colourless to pink |
| 4. | S | remains orange | remains pink |
Answer
R
Reason — Sodium hydroxide is a basic solution. In a basic solution, methyle orange changes from orange to yellow and phenolphthalein changes from colourless to pink.
When electrolysis of molten lead bromide is carried out, the products formed at the respective electrodes are:
| At the positive electrons | At the negative electrode | |
|---|---|---|
| 1. | Bromine | Lead |
| 2. | Bromine | Hydrogen |
| 3. | Lead | Bromine |
| 4. | Lead | Oxygen |
Answer
- At the positive electrode — Bromine
At the negative electrode — Hydrogen
Reason — During electrolysis of molten lead bromide,
At the positive electrode — Dark reddish brown fumes of bromine is evolved
Br1- - 1e- ⟶ Br
Br + Br ⟶ Br2
At the negative electrode — Silver gray metal lead is formed.
Pb2+ + 2e- ⟶ Pb
The following are the structural diagrams of certain hydrocarbons:
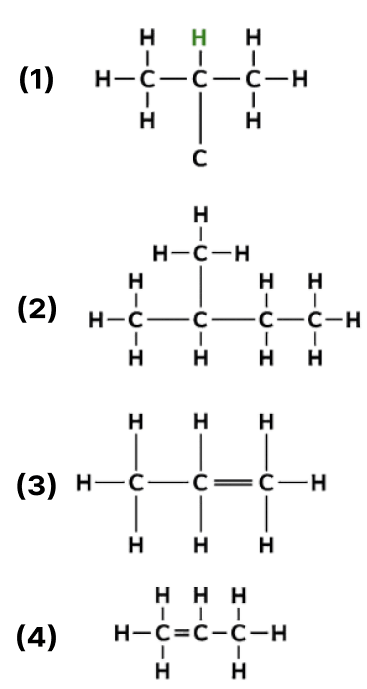
Which two structures are related to each other?
- A and B
- B and C
- C and D
- A and C
Answer
A and C
Reason — Structure A is 2-methylpropane and structure B is n-butane.
Structure A and structure B are isomers of butane, having the molecular formula C4H10.
The electronic configuration of X is 2,8,6. It gains 'Y’ electrons into its valence shell to attain the nearest noble gas electronic configuration and gets converted to an ion Z. X, Y, and Z, respectively, are:
- Sodium, one, electropositive
- Beryllium, two, electronegative
- Oxygen, six, electronegative
- Sulphur, two, electronegative
Answer
Sulphur, two, electronegative
Reason — X has electronic configuration 2, 8, 6. The molecule has total 16 electrons, So, the atomic number is 16. Hence, the element X is Sulphur (S). To achieve the nearest nobel gas configuration of argon(2,8,8), sulphur will gain two electrons. So, Y is 2. Gaining of electrons forms a negative ion. Thus, sulphur will be electronegative.
Which of the following arrangements is INCORRECT as per the property stated against it?
- Li > Be > N > O (Metallic character)
- CI > F > Br > I (Electron gain enthalpy)
- O2- > F - > Mg2+ > Na+(Ionic radii)
- I > Br > CI > F (Number of shells)
Answer
O2- > F - > Mg2+ > Na+(Ionic radii)
Reason —
| Periodic property | Arrangement | Explanation |
|---|---|---|
| Metallic character | Li > Be > N > O | Metallic character decreases across a period from left to right |
| Electron gain enthalpy | CI > F > Br > I | Electron gain enthalpy generally becomes less negative down a group |
| Ionic radii | O2- > F - > Mg2+ > Na+ | Ionic radius of isoelectronic species (same number of electrons), decreases with increasing nuclear charge |
| Number of shells | I > Br > CI > F | Number of shells increases down the group. I has the most shells |
In case of ionic radius, O2- > F - > Mg2+ > Na+ has 10 electrons each, Atomic numbers(Z) of O = 8, F = 9, Na = 11, Mg = 12, Higher the Z, greater is the attraction and smaller is the radius.
The correct order would be, O2- > F - > Na+ > Mg2+.
Baking soda (NaHCO3), when added to vinegar, evolves a gas. Which of these statements is true about the evolution of gas?
I. It turns limewater milky.
II. It extinguishes the burning splinter.
III. It acts as a non-metallic oxide
IV. It has a pungent odour.
- I and IV
- I and II
- I, II and III
- III and IV
Answer
I, II and III
Reason — When baking soda (NaHCO3), added to vinegar (acetic acid), CO2 is evolved. CO2 turns limewater milky, extinguishes the burning splinter and acts as a non-metallic oxide.
CH3COOH + NaHCO3 ⟶ CH3COONa + H2O + CO2 ↑
CO2 has no pungent odour.
The statements below show the results when three metal strips, P, Q, and R, are placed in blue copper sulphate solution.
P — Solution turns green. Q — Solution becomes colourless. R — Solution remains blue.
Which of the following metals could be P, Q, and R?
- P-Al, Q-Zn, R- Fe
- P-Zn, Q-Fe, R- Ag
- P-Fe, Q-Zn, R-Ag
- P- Zn, Q-AI, R– Fe
Answer
P-Fe, Q-Zn, R-Ag
Reason — P is Fe and Q is Zn, because they are more reactive and present above Cu in activity series, so Fe and Zn displace Cu2+ and forms Fe2+ and Zn2+ respectively. Fe2+ gives pale green colour and the blue colour fades when Zn displaces Cu.
R is Ag, as it is less reactive than Cu and cannot displace Cu, so solution stays blue.
Study the below diagram and choose the correct option related to the content given below:
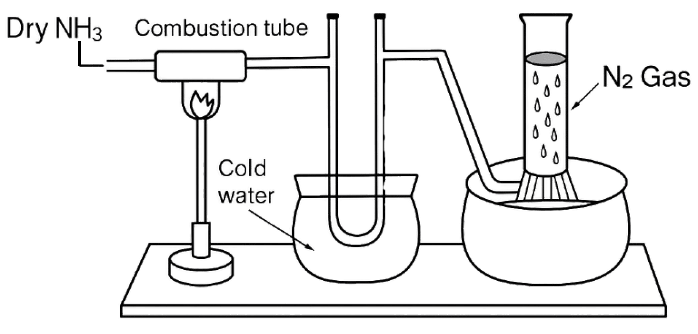
Compound X reacts with ammonia in the combustion tube, which leaves a residue Y. Identify X and Y, as well as the property Z of ammonia demonstrated in this particular reaction.
- X= CuO, Y=black, Z = reducing property.
- X=PbO, Y = yellow, Z=oxidising property.
- X=CuO, Y =yellow, Z =oxidising property.
- X=PbO, Y=black, Z=reducing property.
Answer
X= CuO, Y=black, Z = reducing property.
Reason — Ammonia acts as reducing agent, and reduces heated black solid Copper oxide to give reddish brown Copper metal, water vapour and nitrogen.
2NH3 + 3CuO ⟶ 3Cu + 3H2O + N2 [g]
Assertion (A): Few drops of dilute acid is added to a solution of zinc sulphide, a colourless gas is formed with a rotten egg odour.
Reason (R): Gas formed does not turn moist lead acetate paper silvery black.
- Both A and R are true.
- A and R are true, but R is the correct explanation of A.
- A is true, but R is not the correct explanation of A.
- Both A and R are false.
Answer
A is true, but R is not the correct explanation of A
Reason — When you add dilute acid like HCl to zinc sulphide (ZnS), a colourless H2S gas is formed with the a rotten egg odour. Hence, the assertion (A) is true.
H2S reacts with moist lead acetate paper and forms black PbS (lead sulphide). Hence, reason (R) is not the correct explanation of assertion (A).
Assertion (A): Hall Heroult’s process is used to get pure aluminium from its oxide.
Reason (R): Aluminium generally is not found in aluminium oxide form.
- Both A and R are correct.
- A is correct, but R is not a true explanation of A.
- A is correct, and R is a true explanation of B.
- Both A and R are incorrect.
Answer
Both A and R are correct.
Reason — The Hall-Heroult process is used to extract pure aluminium metal by the electrolytic reduction of aluminium oxide (Al2O3), which is obtained from bauxite by bayer's process. Hence, the assertion (A) is true.
Aluminium is generally not found in aluminium oxide form, aluminum is highly reactive and is found in its main ore bauxite (Al2O3.2H2O). Hence, the reason (R) is correct.
Assertion (A): Alkenes, alkynes and alkanes are examples of homologous series.
Reason (R): Organic compounds of the homologous series have similar structures but different chemical properties.
- Both A and R are true.
- Both A and R are false.
- A is true but R is not the correct explanation of A.
- A is false but R is true.
Answer
A is true but R is not the correct explanation of A.
Reason — Alkenes, alkynes and alkanes are examples of homologous series represented by the same general formula and having a similar structure and similar chemical properties in which the successive compounds differ by a CH2 group. Hence, the assertion (A) is true.
Organic compounds of the homologous series have similar structures and similar chemical properties. Hence, reason (R) is not the correct explanation of assertion (A).
Assertion (A): The atomic mass of oxygen is 16 a.m.u; therefore, its gram atomic mass is 16g.
Reason (R): The atomic mass of an element expressed in grams is called gram atomic mass.
- A is true, and R is the correct explanation of A.
- Both A and R are true, but R is not a true explanation of A.
- Both A and R are false.
- R is false, but A is a true explanation.
Answer
A is true, and R is the correct explanation of A.
Reason — The gram atomic mass is defined as the atomic mass of an element expressed in grams. Thus, the atomic mass of oxygen is 16 a.m.u; therefore, its gram atomic mass is 16g. Hence, the assertion (A) is true.
Reason (R) defines gram atomic mass and why 16 a.m.u of oxygen is same as 16g of gram atomic mass of oxygen.