Chemistry
An alkane with molecular mass 44 is:
- CH4
- C3H8
- C4H10
- C2H6
Organic Chemistry
1 Like
Answer
C3H8
Reason — Let's check the molecular masses:
- CH4 → 12 + 4×1 = 16
- C2H6 → 2×12 + 6×1 = 24 + 6 = 30
- C3H8 → 3×12 + 8×1 = 36 + 8 = 44
- C4H10 → 4×12 + 10×1 = 48 + 10 = 58
So, the alkane with molecular mass 44 is C3H8 (propane).
Answered By
2 Likes
Related Questions
Which of the following will occupy the volume 2.8 litres at S.T.P.?
(Atomic weight: C = 12, O = 16, Cl = 35.5, S = 32)- 2 moles of carbon dioxide
- 7.1 g of chlorine
- 8 g of sulphur dioxide
- 56 g of carbon monoxide
A salt solution which gives a reddish-brown precipitate with NaOH and a white precipitate with BaCl2 solution is:
- CuSO4
- Ca(NO3)2
- Fe2(SO4)3
- FeCl3
The gas evolved in the diagrammatic set up given below turns calcium hydroxide solution milky. The gas evolved is:
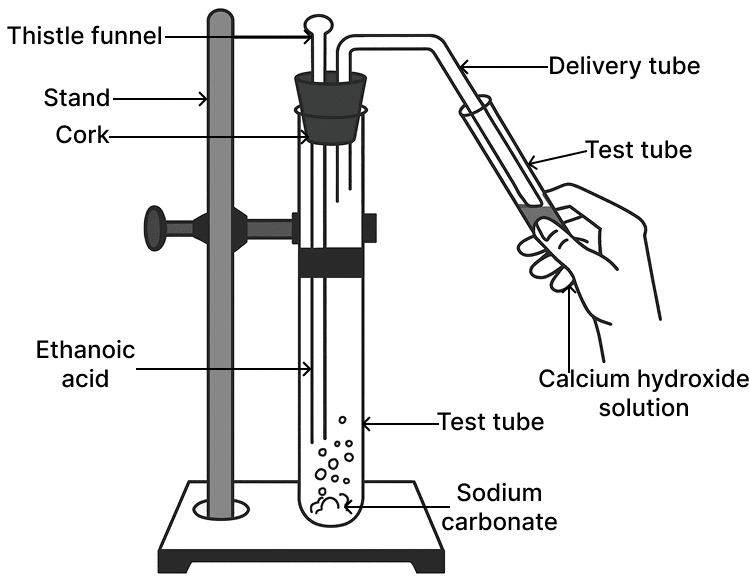
- CH4
- C2H6
- CO2
- SO2
Which gas is evolved when ammonia gas is passed over buff yellow PbO?
- N2O
- NO
- N2
- NO2