Chemistry
Arrange the following according to the instructions given in brackets:
(i) K, Pb, Ca, Zn. (In the increasing order of the reactivity)
(ii) Mg2+, Cu2+, Na1+, H1+ (In the order of preferential discharge at the cathode)
(iii) Li, K, Na, H (In the decreasing order of their ionization potential)
(iv) F, B, N, O (In the increasing order of electron affinity)
(v) Ethane, methane, ethene, ethyne. (In the increasing order of the molecular weight) [H = 1, C = 12]
Periodic Table
ICSE 2019
3 Likes
Answer
(i) Pb < Zn < Ca < K
(ii) Cu2+, H1+, Mg2+, Na1+
Lower the position of the ion, greater the tendency to be liberated at the cathode (or respective electrode).
(iii) H > Li > Na > K
Ionization Potential decreases as we move down the group.
(iv) B < N < O < F
Electron Affinity increases from left to right across a period.
(v) Methane < Ethyne < Ethene < Ethane
Ethane [C2H6] : M.W. = 2[12] + 6[1] = 30
Methane [CH4] : M.W. = 12 + 4[1] = 16
Ethene [C2H4] : M.W. = 2[12] + 4[1] = 28
Ethyne [C2H2] : M.W. = 2[12] + 2[1] = 26
Hence, increasing order of molecular weight : Methane < Ethyne < Ethene < Ethane
Answered By
1 Like
Related Questions
(i) Give the IUPAC name of the following organic compounds:
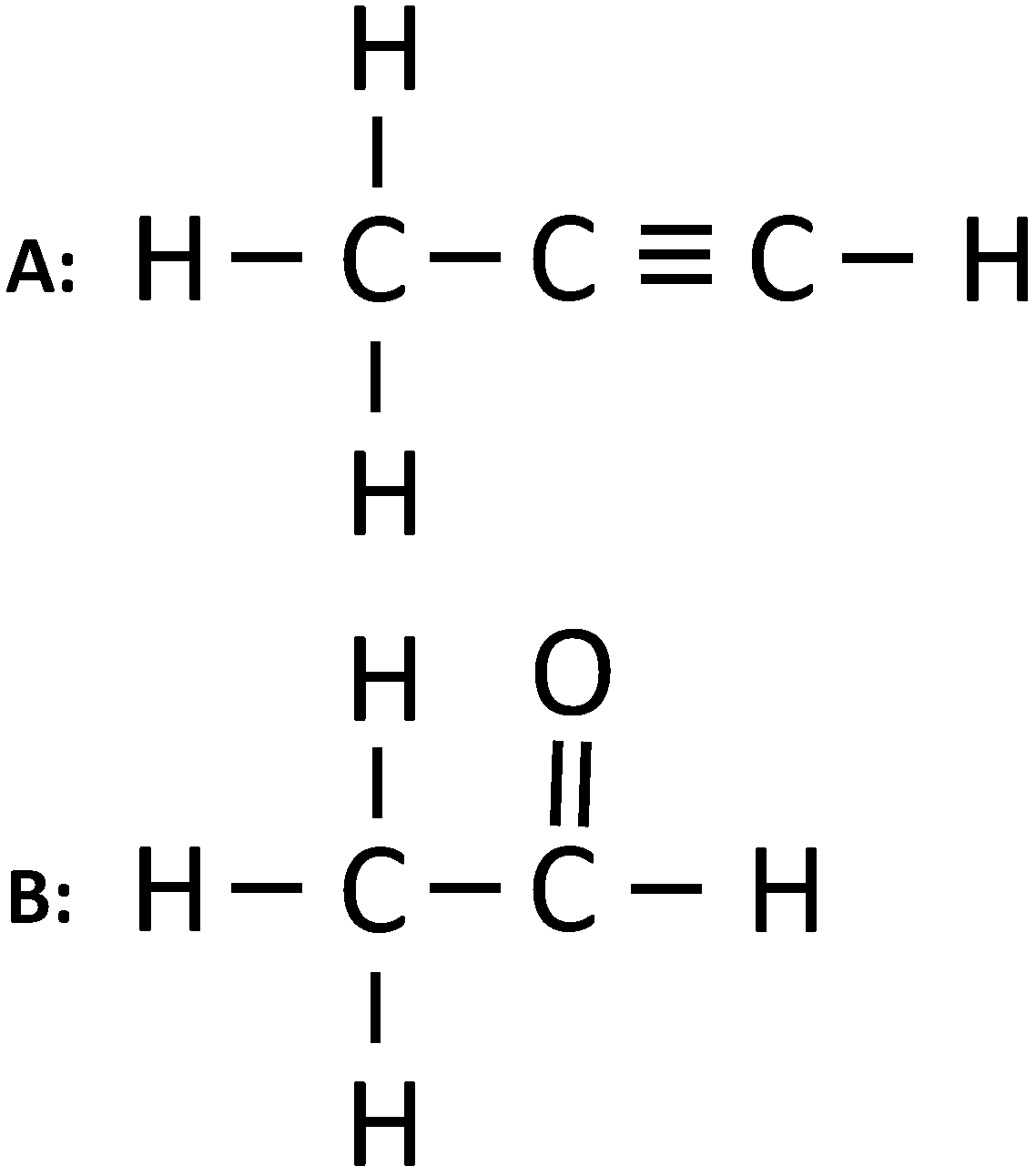
(ii) What is the special feature of the structure of ethyne
(iii) Name the saturated hydrocarbon containing two carbon atoms.
(iv) Give the structural formula of acetic acid.
Give the appropriate term defined by the statements given below:
(i) The formula that represents the simplest ratio of the various elements present in one molecule of the compound.
(ii) The substance that releases hydronium ion as the only positive ion when dissolved in water.
(iii) The tendency of an atom to attract electrons towards itself when combined in a covalent compound.
(iv) The process by which certain ores, specially carbonates, are converted to oxides in the absence of air.
(v) The covalent bond in which the electrons are shared equally between the combining atoms.
Draw the electron dot structure of:
(i) Nitrogen molecule [N = 7]
(ii) Sodium chloride [Na = 11, Cl = 17]
(iii) Ammonium ion [N = 7, H = 1]
The pH values of three solutions A, B, C are given .
Solution A : pH value 12
Solution B : pH value 2
Solution C : pH value 7
Answer the following questions :
(i) Which solution will have no effect on litmus solution.
(ii) Which solution will liberate CO2 when reacted with sodium carbonate.
(iii) Which solution will turn red litmus solution blue.