Chemistry
Assertion (A): In the Contact Process SO3 gas is not directly dissolved in water to obtain sulphuric acid.
Reason (R): Dense fog or misty droplets of sulphuric acid are formed which is difficult to condense.
- Both (A) and (R) are true, and (R) is the correct explanation of (A).
- Both (A) and (R) are true, and (R) is not the correct explanation of (A).
- (A) is true but (R) is false.
- (A) is false but (R) is true.
Answer
Both (A) and (R) are true, and (R) is the correct explanation of (A).
Reason — When sulphur trioxide is brought into direct contact with water,
SO3(g) + H2O(l) ⟶ H2SO4(l)
the reaction is highly exothermic. The heat released vaporises some of the acid and produces a dense fog of minute H2SO4 droplets, which are difficult to condense and collect. Therefore, in the Contact Process the SO3 is first absorbed in concentrated sulphuric acid to form oleum, which is later diluted with water to give the required concentration of H2SO4.
Related Questions
The diagram given below shows the bonding in the covalent molecule AB2.
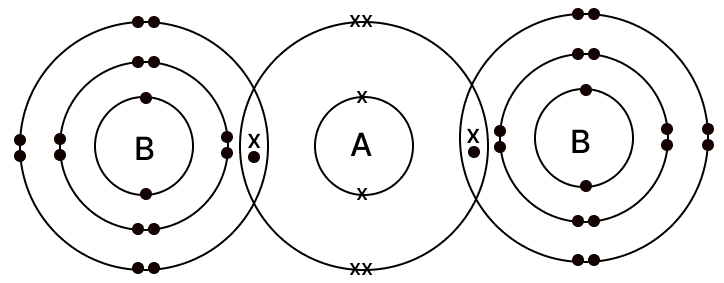
Which option represents the correct electronic configuration of atoms A and B before combining together to form the above molecule?
A B 1 2, 4 2, 8, 6 2 2, 4 2, 8, 7 3 2, 8 2, 8, 8 4 2, 6 2, 8, 7 Which of the following options has all the compounds which are members of the same homologous series?
- CH4, C2H6, C3H8
- CH4, C2H6, C3H6
- C3H4, C3H6, C3H8
- C2H4, C3H6, C4H10
Given below are four ions:
Cl-, Li+, Al3+, K+
Identify the pair of ions which have the same electronic configuration.
[Atomic number: Cl = 17, Li = 3, Al = 13, K = 19]- Cl- & Li+
- Al3+ & K+
- Cl- & K+
- Li+ & K+
Which pair of reactants can be best used to produce lead (II) sulphate?
- Sulphuric acid + Lead
- Sulphuric acid + Lead hydroxide
- Sodium sulphate + Lead nitrate
- Potassium sulphate + Lead oxide