Chemistry
Assertion (A): The tendency of losing electrons increases down the Group.
Reason (R): The most reactive metal is placed at the top of Group 1.
- Both (A) and (R) are true, and (R) is the correct explanation of (A).
- Both (A) and (R) are true, and (R) is not the correct explanation of (A).
- (A) is true but (R) is false.
- (A) is false but (R) is true.
Periodic Table
4 Likes
Answer
(A) is true but (R) is false.
Reason — As we move down any group in the periodic table, atomic size increases and the outermost electron is held less tightly by the nucleus. Therefore the loss of this electron becomes easier, so the tendency to lose electrons increases; Assertion (A) is true. In group 1 the reactivity of metals increases down the group for the same reason. Consequently, the most reactive metal is found at the bottom, not at the top; Reason (R) is false.
Answered By
2 Likes
Related Questions
An organic compound has a vapour density of 22. The molecular formula of the organic compound is: [Atomic weight: C = 12, H = 1]
- CH4
- C2H4
- C2H6
- C3H8
In the reaction given below sulphuric acid acts as a/an:
S + 2H2SO4 ⟶ 3SO2 + 2H2O
- Non-volatile acid
- Dibasic acid
- Oxidising agent
- Reducing agent
The ore that can be concentrated by using magnetic separation:
- Corundum
- Haematite
- Calamine
- Bauxite
The diagram given below shows the bonding in the covalent molecule AB2.
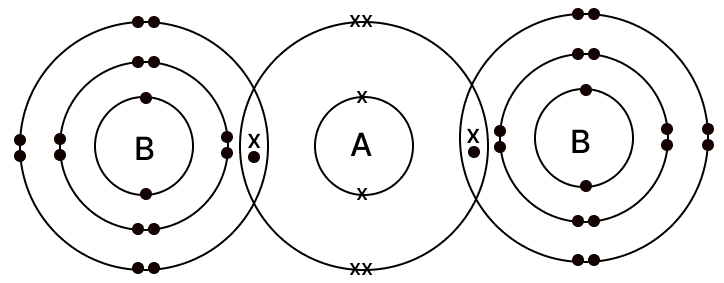
Which option represents the correct electronic configuration of atoms A and B before combining together to form the above molecule?
A B 1 2, 4 2, 8, 6 2 2, 4 2, 8, 7 3 2, 8 2, 8, 8 4 2, 6 2, 8, 7