Physics
Data:
mass =10 g
quantity of heat supplied =120 J
rise in temperature = 10 °C
Statement A : The heat capacity of the substance is 12 J/g.
Statement B : The specific heat capacity of the substance is 1.2 J/gK.
- Only statement A is correct.
- Only statement B is correct.
- Both statements A and B are correct.
- Both statements A and B are incorrect.
Calorimetry
10 Likes
Answer
Only statement B is correct.
Explanation :
Given,
Mass (m) = 10 g
Heat supplied (Q) = 120 J
Temperature rise (ΔT) = 10 °C = 10 K
Statement A is incorrect:
Heat capacity of the sample (C) = = J/K
Here, numerical value is correct but unit of heat capacity is wrong in statement A.
Statement B is correct:
Specific heat capacity (c) = = = 1.2 J/g K
Answered By
4 Likes
Related Questions
…………… of UV > …………… of microwaves
The quantity suitable for filling in both the blanks is:
- speed in vacuum
- speed in a medium
- frequency
- wavelength.
Sumit, a teenager, enjoys playing his music loudly. However, his grandmother consistently lowers the volume of the music player. Analyze the wave to identify which characteristic of the wave has been altered when the grandmother reduces the volume.
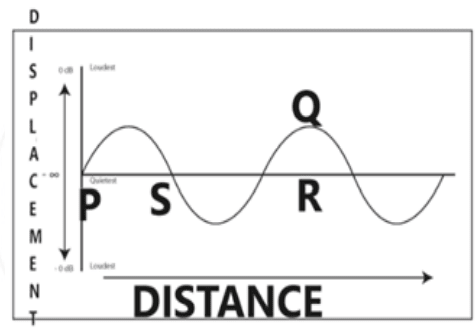
- The length PS becomes more.
- The length SR becomes less.
- The length QR becomes less.
- The length PR becomes less.
Given that the resistivity of Gold and Platinum is 2.1 x 10-8 ohm.m and 10.5 x 10-8 ohm.m, respectively, which is the correct statement?
Statement A : 5 metres of platinum wire will have the same resistance as one metre of gold wire.
Statement B : Gold wire of 1 cm radius will have the same resistance as platinum wire of 5 cm radius.
- Only statement A is correct.
- Only statement B is correct.
- Both statements A and B are correct.
- Both statements A and B are incorrect.
Assertion (A) : A switch will serve the purpose of making and breaking a circuit when it is connected to the neutral wire.
Reason(R) : Neutral wire is at zero potential.
- Both Assertion and Reason are true, and the Reason is the correct explanation of the Assertion
- Both Assertion and Reason are true, but Reason is not the correct explanation of the Assertion
- Assertion is true, but the Reason is false.
- Both Assertion & Reason are false.