Chemistry
Draw an electron dot diagram for the formation of each of the following :
(i) Hydronium ion,
(ii) Ammonium ion,
(iii) Hydroxyl ion.
State the type of bonding present in them.
Chemical Bonding
118 Likes
Answer
(i) Hydronium ion
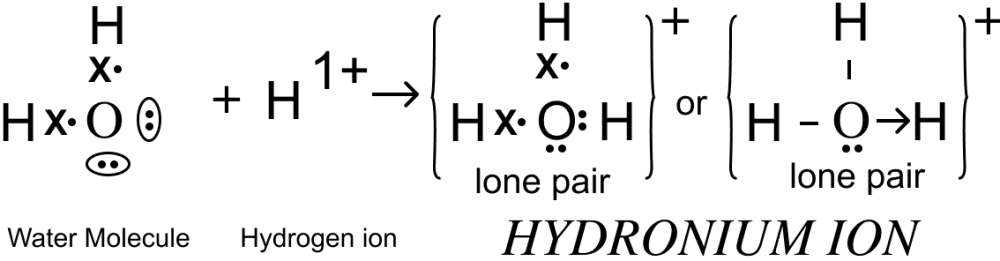
Co-ordinate & Covalent Bond.
(ii) Ammonium ion

Co-ordinate & Covalent Bond.
(iii) Hydroxyl ion

Polar Covalent Bond.
Answered By
74 Likes
Related Questions
A compound is formed between atoms A and B. The electronic configuration of A is 2,8,1 and B is 2,6.
(a) Write the equation of the formation of ions of A and B.
(b) Why do A and B form ions?
(c) Which of the following can form a compound?
(i) A and A (ii) A and B (iii) B and B
Also state the type of bonding present in the compound(d) If A and B combines, which of them will get oxidised?
(e) Draw an electron dot diagram of the ionic compound formed in part (c).
(f) Write the formula of the compound formed in part (c) which has a
(i) high melting and boiling point
(ii) gaseous compound
(iii) good conductor of electricity in molten state.Define a coordinate bond and give the conditions for its formation.
How many atoms of each kind are present in the following molecules : calcium oxide, chlorine, water, carbon tetrachloride ?
How many electrons are required or released by each atom mentioned in (a) to attain the nearest noble gas configuration ?