Chemistry
Element 'X' forms an oxide with the formula X2O3 which is a solid with high melting point. 'X' would most likely be placed in the group of the Periodic Table as:
(a) Na
(b) Mg
(c) Al
(d) SiJustify your answer in the above question (1).
Periodic Table
ICSE Sp 2025
10 Likes
Answer
(c) Al
The formula X2O3 indicates that the element 'X' forms a trivalent oxide. Thus, 'X' has a valency of 3 like that of Al (Valency of Na, Mg and Si are 1, 2 and 4, respectively). In Group 13, the elements typically form oxides with the general formula X2O3, where 'X' represents the element. The oxides of Group 13 elements, such as Al2O3 (aluminum oxide), have high melting points due to the strong ionic bonds between the metal cations and the oxide anions.
Answered By
4 Likes
Related Questions
Abhishek was given a salt 'X' which was white in colour for analysis. On strong heating it produced a yellow residue, a colourless gas and also a reddish-brown gas. The solution of the salt ‘X’ when tested with excess of ammonium hydroxide produced a chalky white insoluble precipitate.
(a) Name the coloured gas evolved when Abhishek heated the salt strongly.
(b) Which cation was present in the sample given to Abhishek?
(c) Identify the salt given to Abhishek for analysis.
Given below in column A is a schematic diagram of the electrolytic reduction of alumina. Identify the parts labelled as A, B and C with the correct options from the Column B.

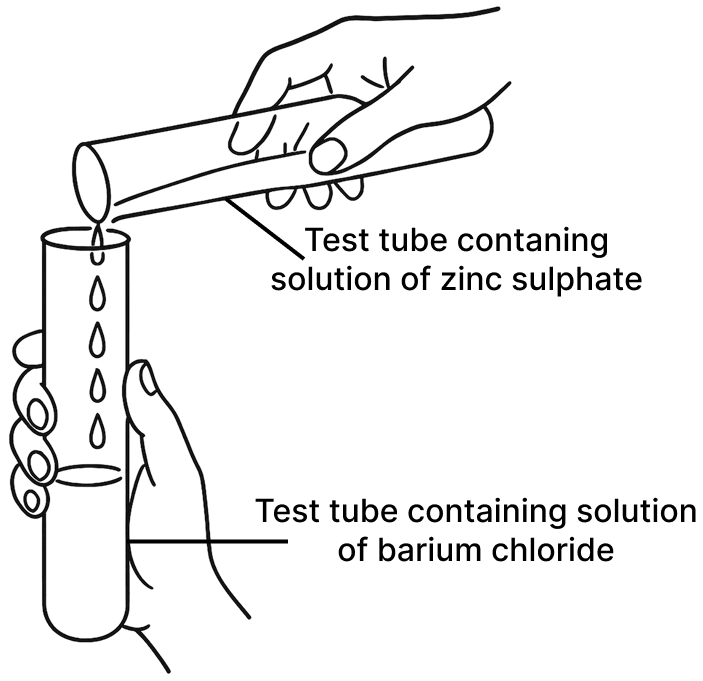
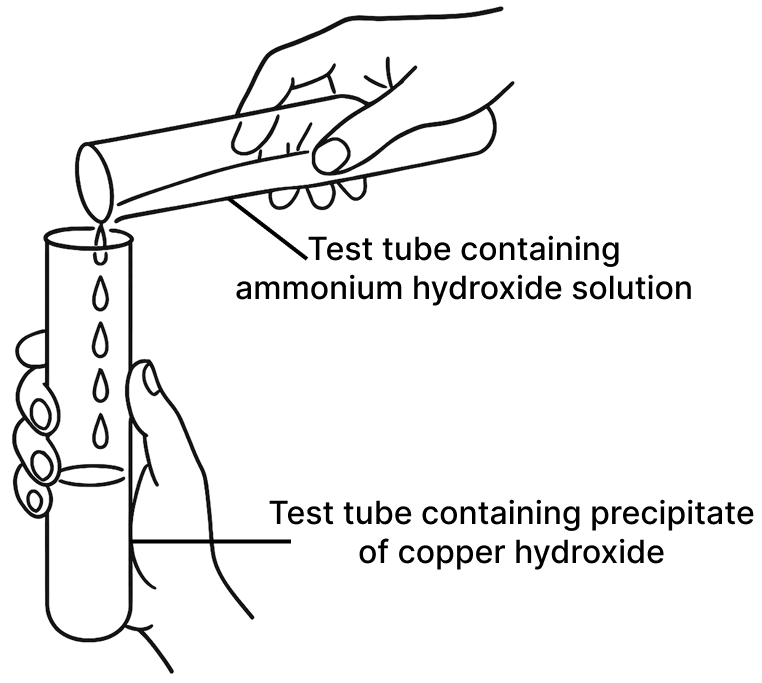
A student was asked to perform two experiments in the laboratory based on the instructions given:
Observe the picture given below and state one observation for each of the Experiments 1 and 2 that you would notice on mixing the given solutions.
(a) Experiment 1
(b) Experiment 2
Copper sulphate solution is electrolysed using copper electrodes.
(a) Which electrode [cathode or anode] is the oxidizing electrode? Why?
(b) Write the equation for the reaction occurring at the above electrode.