Science
An element ‘X’ is stored in kerosene and cannot be extracted from its ore using a reducing agent. ‘X’ forms an ionic compound on reaction with chlorine.
(a) Can we store ‘X’ in water? Give reason to support your answer.
(b) Identify element ‘X’. Name the process used and write the equation for extraction of ‘X’ from its ore.
Answer
(a) No, element ‘X’ cannot be stored in water because it is a highly reactive metal. It will react vigorously with water, producing hydrogen gas and releasing a large amount of heat, which may cause the metal to catch fire or even explode.
Hence, it is stored in kerosene to prevent contact with moisture or air.
(b) Element ‘X’ is sodium (Na).
Sodium is so reactive that it cannot be extracted from its ore by chemical reducing agents like carbon; instead, it is obtained by electrolysis of molten sodium chloride (NaCl) — a process known as electrolytic reduction.
The reactions involved are:
- At cathode: Na+ + e- → Na
- At anode: 2Cl- → Cl2 + 2e-
Related Questions
Assertion (A) : C4H8, C4H6 and C4H10 are members of the same homologous series.
Reason (R) : C4H8, C4H6, C3H4, C3H6, C2H4, C2H2 are unsaturated hydrocarbons.
options
- Both A and R are true, and R is the correct explanation of A.
- Both A and R are true, and R is not the correct explanation of A.
- A is true but R is false.
- A is false but R is true.
The following activity is set-up in the science lab by the teacher. He clamped an aluminium wire on a stand and fixed a pin to the free end of the wire using wax. Then he heated the wire with a burner from the end where the wire is clamped. Students observed the pin fall off.

(a) If the teacher replaces aluminium wire by silver wire, will the students’ observation change? Justify your answer.
(b) Will the aluminium wire melt? Give reason for your answer.
The domes of many building in Europe are made of copper. These domes now appear greenish in colour.
(a) Why do the domes appear greenish though copper is orange-red in colour?
(b) In your opinion, should the copper domes be replaced by iron domes to overcome the problem of change of colour of copper domes?
(c) Domes used to be made from thin sheets of metals. Why did the ancient architects use copper to make domes?
Amrita electrolysed distilled water using the set-up shown in figure 1. She was expecting two gases to be evolved at the anode and cathode respectively.

Suddenly, she realised that the bulb in the circuit did not glow when she used distilled water (figure 2)
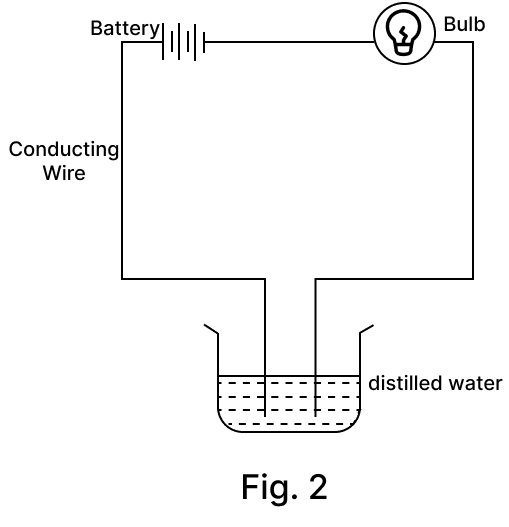
After this realization, she added a substance to the distilled water for electrolysis to take place.
Answer the following questions based on the information given above:
(a) Which gas was she expecting to be formed at the anode and which one at the cathode respectively?
(b) Why did the bulb not glow when Amrita passed electricity through distilled water?
(c) Which substance was added by Amrita to distilled water to get the expected result?