Chemistry
FeS + H2SO4 ⟶ FeSO4 + H2S is an example of:
- Displacement reaction
- Double decomposition reaction
- Decomposition reaction
- Combination reaction
Chemical Reaction
37 Likes
Answer
Double decomposition reaction
Reason — A chemical reaction in which both reactants [compounds] are decomposed to give two new compounds by exchanging their radicals is called a Double decomposition or double displacement reaction.
AB + CD ⟶ AD + CB
Answered By
23 Likes
Related Questions
Which one of these is a chemical change?
- Water changes to steam
- Dissolution of sugar in water
- Combustion of LPG
- Liquefying ammonia
The reaction of neutralization is a :
- Displacement reaction
- Double decomposition
- Combination reaction
- Combination reaction between two compounds
Which of the following statements is/are correct with respect to the figure shown alongside?
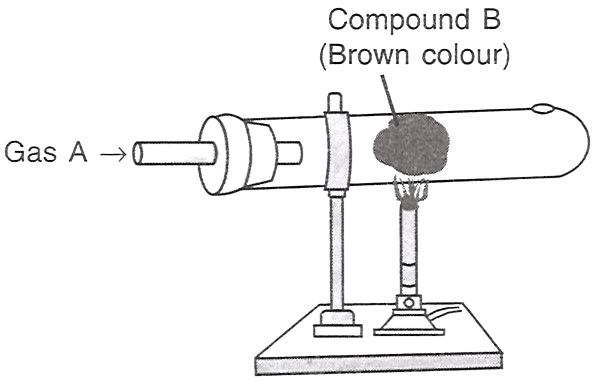
P — A is HCl, B is iron.
Q — A is Cl2, B is copper.
R — A is Cl2, B is iron.
Only P
Only Q
Only R
Both P and R
Aman performed the following four experiments
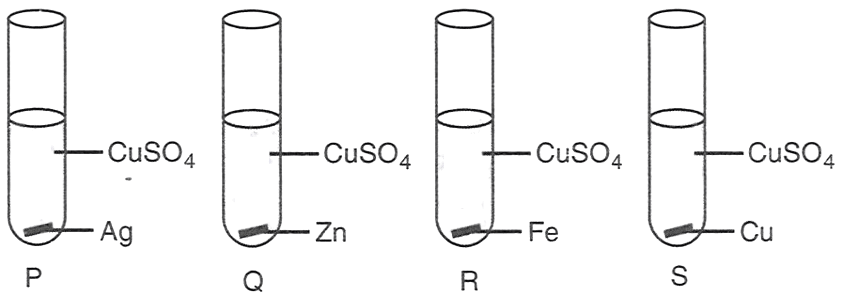
The experiments in which the blue colour of the solution will fade are :
P and Q
P, Q and R
Q and R
Q, R and S