Chemistry
Give balanced equations for the following:
(a) Action of dilute hydrochloric acid on ammonium carbonate.
(b) Oxidation of sulphur with hot concentrated nitric acid.
(c) Reaction of concentrated sulphuric acid with carbon.
Related Questions
Given below are two sets of elements from two different periods. Name the element with the highest ionisation potential in each of the following sets.
(a) Al, Cl, Mg
(b) Ne, O, F
Ammonia gas is passed over heated copper (II) oxide in a combustion tube:
(a) Name the gas evolved.
(b) What will be the colour of the residue that is left in the combustion tube at the end of the reaction?
Rohit took two different salt solutions in test tubes C and D as shown in the figure below. He added dilute HCl to each of the two test tubes. The products formed in the test tubes C and D are silver chloride and lead chloride respectively.
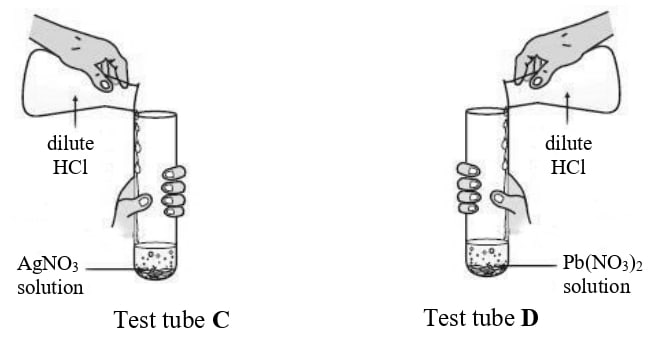
State:
(a) one common observation made by Rohit in both the reactions.
(b) the observations made by him on addition of excess of ammonium hydroxide to the products formed in:
- test tube C
- test tube D
Given below is a diagram showing the placement of five different oxides. With respect to the given diagram answer the following questions:
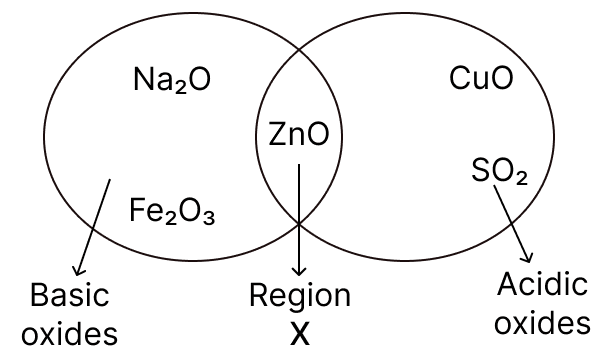
(a) Name the type of oxide represented in region X in the diagram.
(b) Identify the oxide which has been incorrectly placed in the above diagram.
(c) Name the oxide from the above diagram which will form an alkali when dissolved in water.