Chemistry
In the given equation
Zn + Pb2+ ⟶ Zn2+ + Pb
(a) …………… undergoes oxidation.
(b) …………… undergoes reduction.
Related Questions
State the terms for the following:
(a) A substance which when dissolved in water forms hydronium ion as the only positive ion.
(b) A type of covalent bond in which electrons are shared equally between the combining atoms.
(c) The process by which a base metal is coated with another metal, either to protect the metal or to give it an attractive appearance.
(d) The type of reaction characteristic for alkanes.
(e) The substance which oxidises the other substance and itself gets reduced.
(a) Draw the structural diagram for the following organic compounds:
- bromoethane
- methanal
- but-2-yne
(b) Give IUPAC name for the following organic compounds:
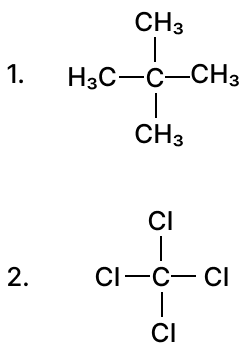
Justify the following statements:
(a) As one moves down a group, the reducing property of elements increases.
(b) Aluminium oxide cannot be reduced by carbon monoxide.
Arrange the following as per the instructions given in the brackets:
(a) Mg, S, Si, P [decreasing order of atomic size]
(b) Cl, I, Br, F [increasing electronegativity]
(c) K, Na, Rb, Li [decreasing metallic character]