Physics
Heat capacity of a body is the :
- energy needed to melt a body without change in its temperature
- energy needed to raise the temperature of a body by 1°C
- increase in volume of the body when its temperature increases by 1°C
- total amount of internal energy that is constant
Calorimetry
1 Like
Answer
energy needed to raise the temperature of a body by 1°C
Reason — The heat capacity of a body is the amount of heat energy required to raise its temperature by 1°C (or 1 K).
Answered By
1 Like
Related Questions
According to the old convention, the colour of the earth wire is :
- black
- green
- yellow
- red
Current is flowing through a coil as shown in the figure. Which one of the given figures will correctly depict the magnetic polarity and the direction of the lines of force along the axis of the coil.
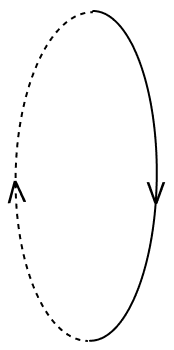
- 1.
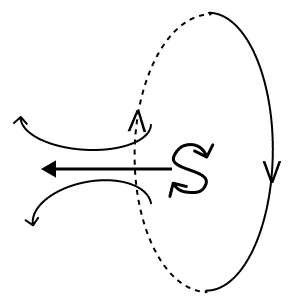
- 2.
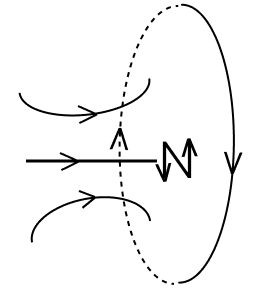
- 3.
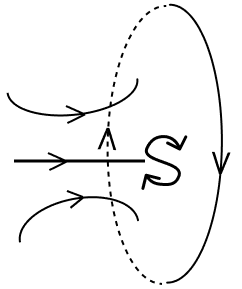
- 4.
The amount of heat energy required to melt a given mass of a substance at its melting point without any rise in its temperature is called as the :
- specific heat capacity
- specific latent heat of fusion
- latent heat of fusion
- specific latent heat of freezing
A nucleus of an atom consists of 146 neutrons and 95 protons. It decays after emitting an alpha particle. How many protons and neutrons are left in the nucleus after an alpha emission?
- protons = 93, neutrons = 142
- protons = 95, neutrons = 144
- protons = 93, neutrons = 144
- protons = 95, neutrons = 142