Chemistry
(i) Calculate:
The number of moles in 12g of oxygen gas. [O = 16]
The weight of 1022 atoms of carbon.
[C = 12, Avogadro’s No. = 6 x 1023]
(ii) Molecular formula of a compound is C6H18O3. Find its empirical formula.
Stoichiometry
ICSE 2019
12 Likes
Answer
(i) Gram molecular mass of oxygen = 32 g = 1 mole
32 g = 1 mole
∴ 12 g = x 12 = 0.375 moles.
6 x 1023 atoms of carbon weigh = 12 g
∴ 1022 atoms of carbon will weigh = x 1022 = 0.2 g.
Hence, number of moles = 0.375 moles and weight of 1022 atoms of carbon = 0.2 g.
(ii) Molecular formula of the compound is C6H18O3
Molecular formula = n x (Empirical Formula)
C6H18O3 can be written as 3(C2H6O)
∴ Molecular formula = 3 x (C2H6O)
∴ Empirical Formula of the compound is C2H6O
Answered By
5 Likes
Related Questions
Write a balanced chemical equation for each of the following reactions:
(i) Reduction of copper (II) oxide by hydrogen.
(ii) Action of dilute sulphuric acid on sodium hydroxide.
(iii) Action of dilute sulphuric acid on zinc sulphide.
(iv) Ammonium hydroxide is added to ferrous sulphate solution.
(v) Chlorine gas is reacted with ethene.
State one observation for each of the following:
(i) Concentrated nitric acid is reacted with sulphur.
(ii) Ammonia gas is passed over heated copper (II) oxide.
(iii) Copper sulphate solution is electrolysed using copper electrodes.
(iv) A small piece of zinc is added to dilute hydrochloric acid.
(v) Lead nitrate is heated strongly in a test tube.
(i) Give the IUPAC name of the following organic compounds:
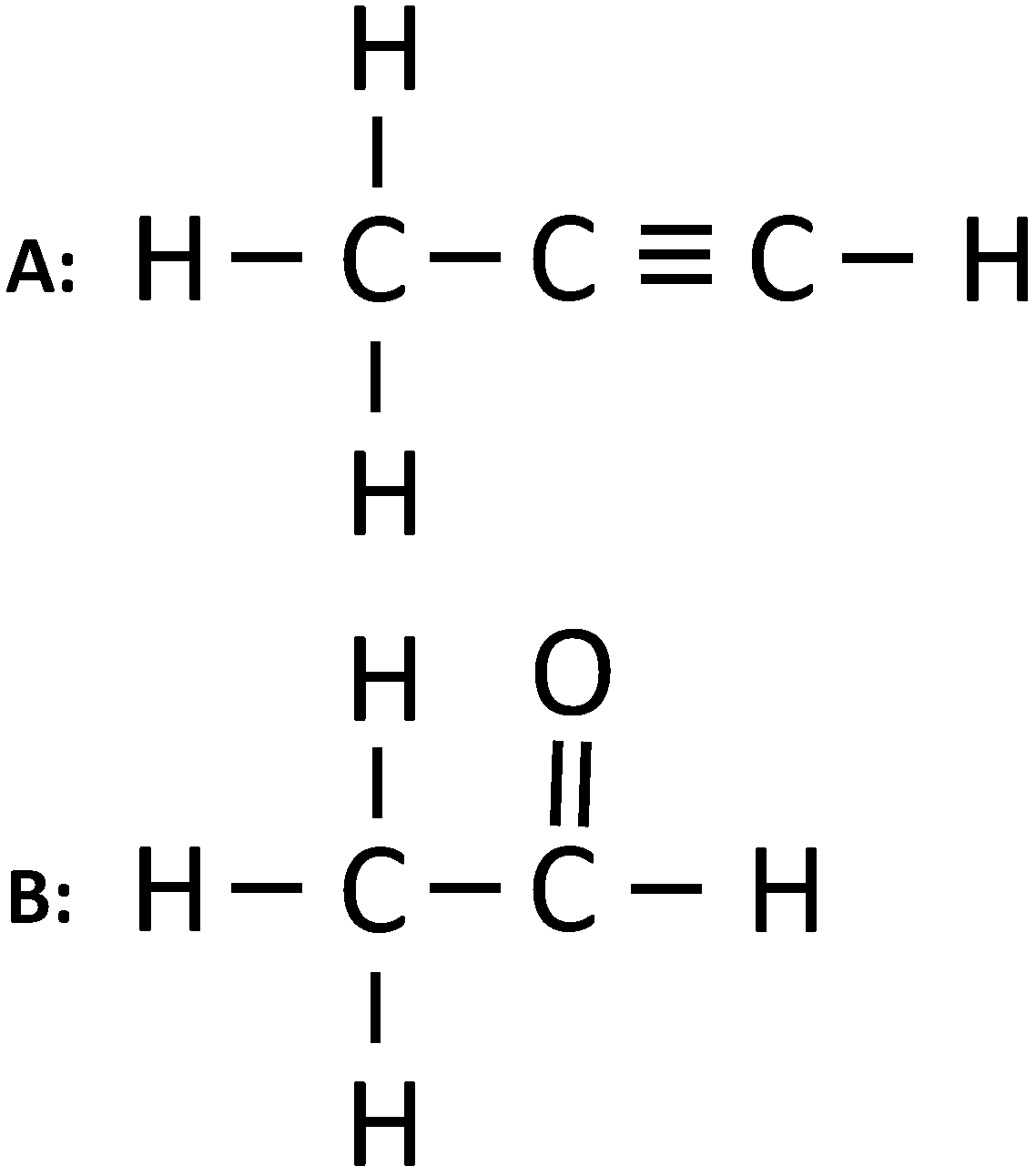
(ii) What is the special feature of the structure of ethyne
(iii) Name the saturated hydrocarbon containing two carbon atoms.
(iv) Give the structural formula of acetic acid.
Give the appropriate term defined by the statements given below:
(i) The formula that represents the simplest ratio of the various elements present in one molecule of the compound.
(ii) The substance that releases hydronium ion as the only positive ion when dissolved in water.
(iii) The tendency of an atom to attract electrons towards itself when combined in a covalent compound.
(iv) The process by which certain ores, specially carbonates, are converted to oxides in the absence of air.
(v) The covalent bond in which the electrons are shared equally between the combining atoms.