Chemistry
Justify the following statements:
(a) As one moves down a group, the reducing property of elements increases.
(b) Aluminium oxide cannot be reduced by carbon monoxide.
Answer
(a) Reducing property means the tendency to lose electrons, On moving down a group atomic size increases. Outer electrons are farther from the nucleus and less strongly attracted. Hence, as we move down the group elements lose electrons more readily, making them stronger reducing agents.
(b) Aluminium oxide is highly stable and aluminium has a greater affinity for oxygen than carbon, so CO is not strong enough to reduce Al2O3.
Related Questions
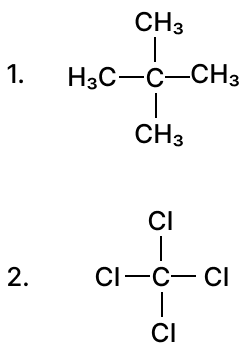
(a) Draw the structural diagram for the following organic compounds:
- bromoethane
- methanal
- but-2-yne
(b) Give IUPAC name for the following organic compounds:
In the given equation
Zn + Pb2+ ⟶ Zn2+ + Pb
(a) …………… undergoes oxidation.
(b) …………… undergoes reduction.
Arrange the following as per the instructions given in the brackets:
(a) Mg, S, Si, P [decreasing order of atomic size]
(b) Cl, I, Br, F [increasing electronegativity]
(c) K, Na, Rb, Li [decreasing metallic character]
Harsh performed the following experiments in the laboratory. State one significant observation made by Harsh when:
(a) he added concentrated sulphuric acid to blue vitriol.
(b) he passed ammonia gas over heated PbO.
(c) sodium hydroxide solution was added to CuSO4 solution by him.