Chemistry
Match the Column A (showing the properties of H2SO4) with Column B (showing the reaction of H2SO4)
| Column A Properties of H2SO4 | Column B Reaction of H2SO4 |
|---|---|
| (a) Acidic property | 1. C12H22O11 + nH2SO4 ⟶ 12C + 11H2O + nH2SO4 |
| (b) Dehydrating property | 2. S + 2H2SO4 ⟶ 3SO2 + 2H2O |
| (c) Non-volatile acid | 3. CaO + H2SO4 ⟶ CaSO4 + H2O |
| (d) Oxidizing agent | 4. NaCl + H2SO4 ⟶ NaHSO4 + HCl |
Sulphuric Acid
5 Likes
Answer
| Column A Properties of H2SO4 | Column B Reaction of H2SO4 |
|---|---|
| (a) Acidic property | 3. CaO + H2SO4 ⟶ CaSO4 + H2O |
| (b) Dehydrating property | 1. C12H22O11 + nH2SO4 ⟶ 12C + 11H2O + nH2SO4 |
| (c) Non-volatile acid | 4. NaCl + H2SO4 ⟶ NaHSO4 + HCl |
| (d) Oxidizing agent | 2. S + H2SO4 ⟶ 3SO2 + 2H2O |
Answered By
2 Likes
Related Questions
Choose the letters L, M, N, O & P to match the description (a) to (c) given below:
[L – Ammonia, M – Nitrogen, N – Hydrogen sulphide, O – Hydrogen chloride gas, P – Nitrogen dioxide]
(a) When this gas comes in contact with ammonia dense white fumes are seen.
(b) The gas that turns moist lead acetate paper silvery black.
(c) The gas produced on heating lead nitrate.
Smith wrote the following statements incorrectly. Insert a word to correct the statements.
(a) Lead bromide conducts electricity.
(b) Copper reacts with nitric acid to form nitrogen dioxide gas.
(c) Bromoethane reacts with sodium hydroxide to produce ethanol and sodium bromide.
Calcium oxide is a drying agent which removes water vapour. A student wanted to collect a dry sample of the hydrogen chloride gas produced. The student set up the apparatus as shown below but was unsuccessful in collecting any gas.
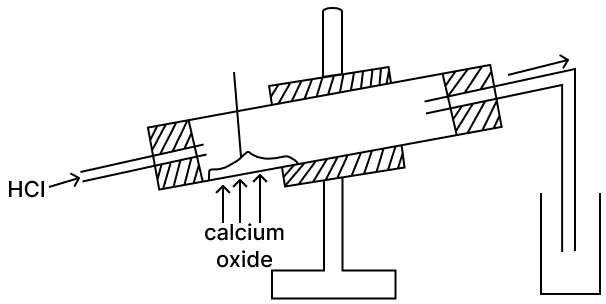
(a) What mistake did the student make?
(b) What change should be made by the student in order to collect the dry HCl gas?
Select the correct answer from the options given in the brackets:
(a) The ion which is discharged at the cathode during the electrolysis of CuSO4 solution using copper electrodes. [Cu2+, OH-, SO42-, H+]
(b) During electroplating of an article with Ag using sodium argentocyanide as an electrolyte, the anode is made of. [Cu, Ag, Pt, Na]