Science
Sara took 2 mL of dilute NaOH solution in a test tube and added two drops of phenolphthalein solution to it. The solution turned pink in colour. She added dilute H2SO4 to the above solution drop by drop until the solution in the test tube became colourless. 40 drops of dilute H2SO4 were used for the change in colour from pink to colourless. When Sara added a drop of NaOH to the solution, the colour changed back to pink again.
Sara now tried the activity with different volumes of NaOH and recorded her observation in the table given below :
| S. No. | Volume of dil. NaOH taken (mL) | Drops of dil. H2SO4 used |
|---|---|---|
| 1 | 2 | 20 |
| 2 | 3 | 30 |
| 3 | 4 | 40 |
Answer the following questions based on the above information:
(a) If Sara used concentrated H2SO4 in place of dilute H2SO4, how many drops will be required for the change in colour to be observed?
- 40
- < 40
- > 40
Justify your answer.
(b) Sara measured 20 drops of dil. H2SO4 and found its volume to be 1 mL. If Sara observed a change in colour of NaOH solution by using 3 mL of H2SO4, how many mL of NaOH did she add to the test tube initially?
OR
Sara takes 10 drops of dilute H2SO4 in the test tube and adds two drops of phenolphthalein solution to it. Then she adds NaOH dropwise. Sara observes a change in colour after adding 20 drops of NaOH. What change in colour would she observe and why?
(c) Write a balanced chemical equation for the reaction taking place in the above experiment. Which of the following is true and why? The reaction is a
- neutralisation and double displacement reaction
- neutralisation and precipitation reaction
- precipitation and double displacement reaction
- neutralisation, double displacement as well as precipitation reaction.
Acids Bases Salts
2 Likes
Answer
(a) < 40 drops.
Concentrated H2SO4 provides more H+ ions per drop than dilute acid, so fewer drops are needed to neutralise the same amount of NaOH and discharge the pink colour of phenolphthalein.
(b) From Sara’s note, 20 drops = 1 mL.
If she used 3 mL H2SO4, that is 60 drops and her table shows the drops of acid are proportional to the mL of NaOH (20 drops per 2 mL NaOH → 10 drops per 1 mL NaOH).
Thus 60 drops of acid would neutralise 6 mL of NaOH.
OR
If she starts with dilute H2SO4 plus phenolphthalein and then adds NaOH dropwise, the solution will change from colourless to pink when the solution becomes basic, because phenolphthalein is colourless in acid and pink in base.
(c) Balanced equation:
2NaOH + H2SO4 → Na2SO4 + 2H2O
This is a neutralisation and double displacement reaction because the acid and base react to form salt + water (neutralisation). Ions exchange partners (Na+ with H+/SO42-), so it’s double displacement. It is not a precipitation reaction because sodium sulphate (Na2SO4) is soluble in water.
Answered By
1 Like
Related Questions
Amrita electrolysed distilled water using the set-up shown in figure 1. She was expecting two gases to be evolved at the anode and cathode respectively.

Suddenly, she realised that the bulb in the circuit did not glow when she used distilled water (figure 2)
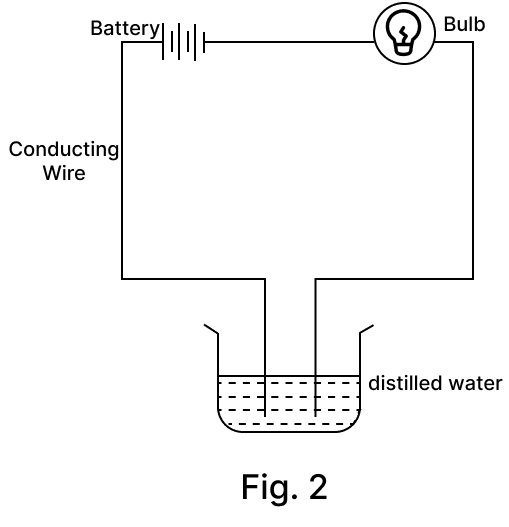
After this realization, she added a substance to the distilled water for electrolysis to take place.
Answer the following questions based on the information given above:
(a) Which gas was she expecting to be formed at the anode and which one at the cathode respectively?
(b) Why did the bulb not glow when Amrita passed electricity through distilled water?
(c) Which substance was added by Amrita to distilled water to get the expected result?
Identify the type of reaction :
(a)
(b)
(c)
A hydrocarbon with the formula CxHy undergoes complete combustion as shown in the following equation :
2CxHy + 9O2 → 6CO2 + 6H2O.
(a) What are the values of ‘x’ and ‘y’?
(b) Give the chemical (IUPAC) name of the hydrocarbon.
(c) Draw its electron dot structure.
(d) Name the alcohol which on heating with conc. H2SO4 will produce the above hydrocarbon CxHy.
(e) Write a balanced chemical equation for the reaction of CxHy with hydrogen gas in presence of Nickel.
The electronic structures of atoms P and Q are shown below :

Based on the information given above, answer the following questions:
(a) If P and Q combine to form a compound, what type of bond is formed between them?
(b) Give the chemical formula of the compound formed.
(c) The compound so formed is dissolved in water. Is the resultant solution acidic or basic in nature? Justify your answer.
(d) Write the chemical equation for the reaction between ‘Q’ and ethanol.
(e) What will be the formula of the compound formed when ‘P’ undergoes bonding with carbon?