Chemistry
Smith wrote the following statements incorrectly. Insert a word to correct the statements.
(a) Lead bromide conducts electricity.
(b) Copper reacts with nitric acid to form nitrogen dioxide gas.
(c) Bromoethane reacts with sodium hydroxide to produce ethanol and sodium bromide.
Answer
(a) Molten lead bromide conducts electricity.
Reason — Solid lead bromide is a non-conductor of electricity since it's ions are not free but held together by an electrostatic force of attraction. In molten state the ions become free and hence conduct electricity.
(b) Copper reacts with concentrated nitric acid to form nitrogen dioxide gas.
Reason — When copper reacts with dilute nitric acid it will form nitric oxide(NO) not nitrogen dioxide.
(c) Bromoethane reacts with aqueous sodium hydroxide to produce ethanol and sodium bromide.
Reason — Alcohol is prepared by the hydrolysis of alkyl halides (haloalkanes) on reaction with aqueous NaOH. Hydrolysis of bromoethane occurs with aqueous NaOH, not anhydrous/alcoholic NaOH.
Related Questions
Harsh performed the following experiments in the laboratory. State one significant observation made by Harsh when:
(a) he added concentrated sulphuric acid to blue vitriol.
(b) he passed ammonia gas over heated PbO.
(c) sodium hydroxide solution was added to CuSO4 solution by him.
Choose the letters L, M, N, O & P to match the description (a) to (c) given below:
[L – Ammonia, M – Nitrogen, N – Hydrogen sulphide, O – Hydrogen chloride gas, P – Nitrogen dioxide]
(a) When this gas comes in contact with ammonia dense white fumes are seen.
(b) The gas that turns moist lead acetate paper silvery black.
(c) The gas produced on heating lead nitrate.
Match the Column A (showing the properties of H2SO4) with Column B (showing the reaction of H2SO4)
Column A
Properties of H2SO4Column B
Reaction of H2SO4(a) Acidic property 1. C12H22O11 + nH2SO4 ⟶ 12C + 11H2O + nH2SO4 (b) Dehydrating property 2. S + 2H2SO4 ⟶ 3SO2 + 2H2O (c) Non-volatile acid 3. CaO + H2SO4 ⟶ CaSO4 + H2O (d) Oxidizing agent 4. NaCl + H2SO4 ⟶ NaHSO4 + HCl Calcium oxide is a drying agent which removes water vapour. A student wanted to collect a dry sample of the hydrogen chloride gas produced. The student set up the apparatus as shown below but was unsuccessful in collecting any gas.
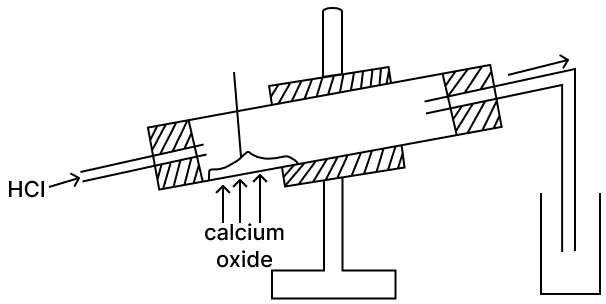
(a) What mistake did the student make?
(b) What change should be made by the student in order to collect the dry HCl gas?