Physics
Some common salt is added to ice. It is found that temperature of mixture drops below 0°C. Explain the fact and where is this used?
Related Questions
(a) The given figure shows three resistors. Find the combined resistance.

(b) Which part of an electrical appliance is earthed?
The diagram below shows a coil connected to the centre zero galvanometer.

What polarity is induced at point X when
(a) the magnet is moved towards the coil
(b) the magnet is moved away from the coil?
Suggest one effective process for the safe disposal of nuclear waste.
A boy uses monochromatic green light to find the refractive index. When a ray of monochromatic green light enters in a liquid medium from air medium as shown in the figure given below, the angle 1 is 45° and angle 2 is 30°.
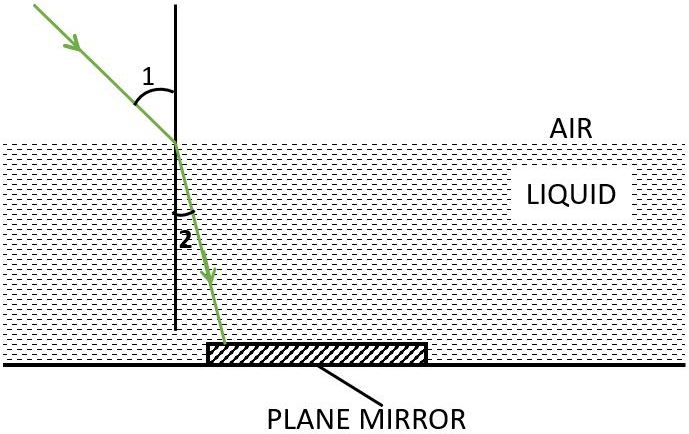
(a) Determine the refractive index of the liquid.
(b) Represent in the diagram showing the path of the ray after it strikes the mirror and re-enters air. Mark in the diagram wherever necessary.
(c) Draw the diagram again, if plane mirror becomes normal to the refracted ray inside the liquid. Name the principle used.