Chemistry
The following table shows the electronic configuration of the elements W, X, Y, Z :
| Element | W | X | Y | Z |
| Electronic Configurations | 2, 8, 1 | 2, 8, 7 | 2, 5 | 1 |
Answer the following questions based on the table above:
(i) What type of bond is formed between:
- W and X
- Y and Z
(ii) What is the formula of the compound formed between :
- X and Z
- W and X
Chemical Bonding
39 Likes
Answer
(i) Type of bond formed
(a) W (2,8,1) and X (2,8,7) — Electrovalent bond is formed as W donates 1 electron and X takes up that electron and both attain a stable octet.
(b) Y (2, 5) and Z (1) — Covalent bond as electron sharing takes place between the two elements. The valency of Z element is 1 and that of Y is 3. Z needs one electrons to attain stable duplet structure of nearest noble gas - He [2] and Y needs three electrons to attain stable octet structure of nearest noble gas - Ne [2,8]
One Y atom shares three electron pairs one with each of the three atoms of Z such that Z acquires a duplet configuration and Y attain a stable octet configuration resulting in the formation of three single covalent bonds.
(ii) Formula of the compound formed
(a) X (2,8,7) and Z (1) — XZ as X needs one 1 electron to complete it's octet and Z needs needs one electron to complete it's duplet hence, they both share one electron pair and forms ZX compound.
(b) W (2,8,1) and X (2,8,7) — WX as W has the tendency to donate one electron and gain stability whereas X has the tendency to gain one electron to be stable. Hence, W donates one electron and X takes it and form WX compound.
Answered By
29 Likes
Related Questions
Compare carbon tetrachloride and sodium chloride with regard to solubility in water and electrical conductivity.
Draw an electron dot diagram of the structure of — hydronium ion. State the type of bonding present in it.
By drawing an electron dot diagram, show the lone pair effect leading to the formation of — ammonium ion from ammonia gas and hydrogen ion.
Study the extract of the Periodic table given below and answer the questions that follow. Give the alphabet corresponding to the element in question: DO NOT repeat an element.
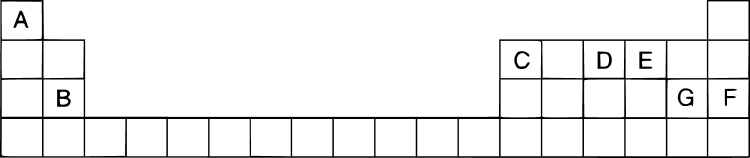
(a) Which element forms an electrovalent compound with G?
(b) The ion of which element will migrate towards the cathode during electrolysis?
(c) Which non metallic element has the valency 2?
(d) Which is the inert gas?