Chemistry
Three different solutions X (sodium chloride solution), Y (acetic acid) and Z (sugar solution) were used for electrolysis by a student. When the circuit was completed, he noticed that the bulb glowed in the electrolytic cell containing:
- X & Y
- Y & Z
- Z & X
- X, Y & Z
Electrolysis
3 Likes
Answer
X & Y
Reason — X (NaCl solution) is an electrolyte, it contains free Na+ and Cl- ions, so it conducts electricity well.
Y (acetic acid) is a weak electrolyte, it partially ionises to give H+ and CH3COO- , so it can conduct where the bulb may glow dimmer than with a strong electrolyte. Whereas, Z (sugar solution) contains molecular sucrose which does not dissociate into ions.
Answered By
1 Like
Related Questions
The gas evolved in the diagrammatic set up given below turns calcium hydroxide solution milky. The gas evolved is:
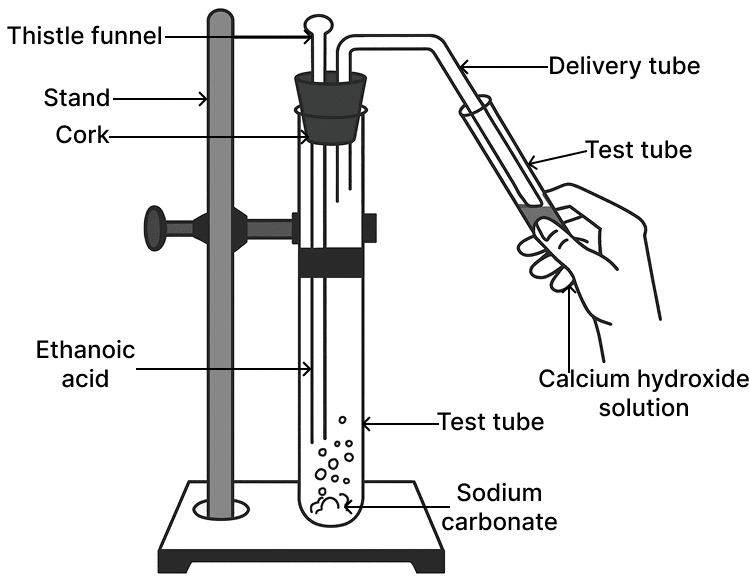
- CH4
- C2H6
- CO2
- SO2
Which gas is evolved when ammonia gas is passed over buff yellow PbO?
- N2O
- NO
- N2
- NO2
An element X has an electronic configuration 2, 2. The compound formed when X combines with oxygen is most likely to be:
- a compound with a low melting point.
- a gas that dissolves in water to form an electrolyte.
- a good conductor in both solid and molten state.
- an ionic solid.
If an element has a low ionisation potential, it is most likely to be a:
- metal
- non-metal
- metalloid
- inert gas