Chemistry
X [2, 8, 7] and Y [2, 8, 2] are two elements. Using this information complete the following:
(a) …………… is the metallic element.
(b) Metal atoms tend to have a maximum of …………… electrons in the outermost shell.
(c) …………… is the reducing agent.
Chemical Bonding
ICSE Sp 2025
10 Likes
Answer
(a) Y is the metallic element.
(b) Metal atoms tend to have a maximum of 3 electrons in the outermost shell.
(c) Y is the reducing agent.
Reason
(a) As Y has two valence electrons, hence it is a metallic element.
(b) Metal atoms tend to have a maximum of 3 electrons in the outermost shell.
(c) The reducing agent is Y because it can easily lose its two valence electrons to form a stable ion, thus reducing other substances by donating electrons.
Answered By
6 Likes
Related Questions
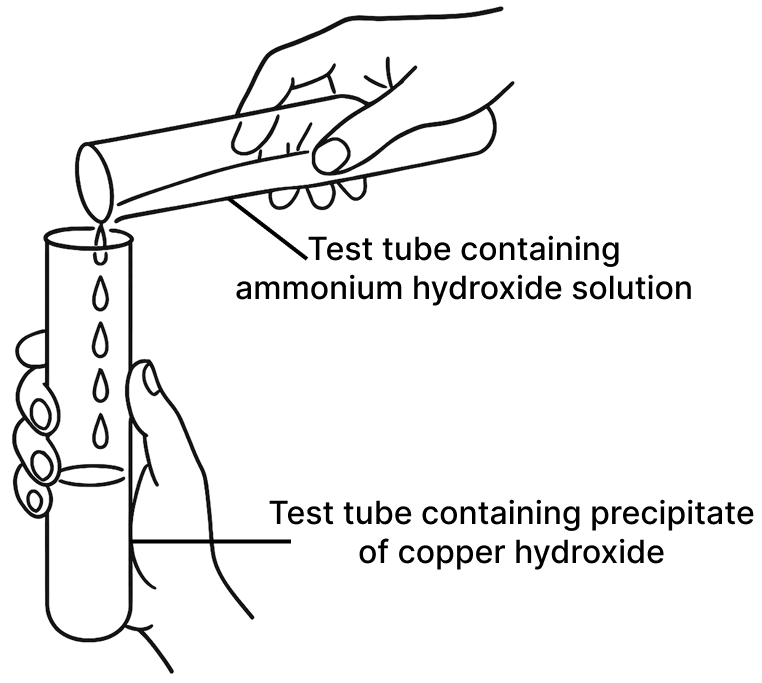
A student was asked to perform two experiments in the laboratory based on the instructions given:
Observe the picture given below and state one observation for each of the Experiments 1 and 2 that you would notice on mixing the given solutions.
(a) Experiment 1
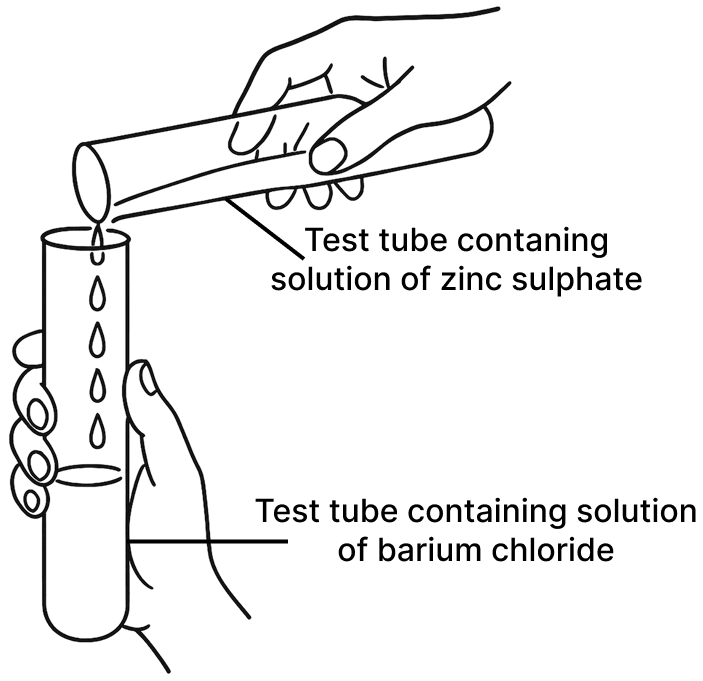
(b) Experiment 2
Copper sulphate solution is electrolysed using copper electrodes.
(a) Which electrode [cathode or anode] is the oxidizing electrode? Why?
(b) Write the equation for the reaction occurring at the above electrode.
One variety of household fuel is a mixture of propane (60%) and butane (40%). If 20 litres of this mixture is burnt, find the total volume of carbon dioxide added to the atmosphere. The combination reactions can be represented as:
C3H8 + 5O2 ⟶ 3CO2 + 4H2O
2C4H10 + 13O2 ⟶ 8CO2 + 10H2O
Rohit has solution X, Y and Z that has pH 2, 7 and 13 respectively.
Which solution:(a) will liberate sulphur dioxide gas when heated with sodium sulphite
(b) will liberate ammonia gas when reacted with ammonium chloride
(c) will not have any effect on litmus paper?