Electrolysis of water is a decomposition reaction. The mass ratio (MH : MO) of hydrogen and oxygen gases liberated at the electrodes during electrolysis of water is :
options
- 8 : 1
- 2 : 1
- 1 : 2
- 1 : 8
Answer
1 : 8
Reason — Electrolysis of water is a decomposition reaction, because water (H2O) breaks down into its constituent elements—hydrogen and oxygen—when an electric current passes through it.
During the process, hydrogen gas is released at the cathode and oxygen gas is released at the anode.
According to the chemical equation
2H2O → 2H2 + O2
the ratio of the number of molecules (or volumes) of hydrogen to oxygen formed is 2 : 1.
However, since the molecular mass of hydrogen (H2) is 2 and that of oxygen (O2) is 32, the mass ratio of hydrogen to oxygen becomes (2 × 2) : (1 x 32) = 4 : 32 = 1 : 8.
Hence, the mass ratio (MH : MO) of hydrogen and oxygen gases liberated during the electrolysis of water is 1 : 8.
The products formed when Aluminium and Magnesium are burnt in the presence of air respectively are :
options
- Al3O4 and MgO2
- Al2O3 and MgO
- Al3O4 and MgO
- Al2O3 and MgO2
Answer
Al2O3 and MgO
Reason —
When aluminium burns in air, it forms aluminium oxide (Al2O3) and when magnesium burns in air, it forms magnesium oxide (MgO).
The following table shows the pH values of four solutions A, B, C and D on a pH scale :

The solutions A, B, C and D respectively are of a
options
- Strong acid, weak acid, neutral, strong base
- Weak acid, neutral, weak base, strong base
- Weak acid, neutral, strong base, weak base
- Weak acid, neutral, strong base, strong acid
Answer
Weak acid, neutral, strong base, strong acid
Reason — According pH scale :
- A (pH = 5) → weak acid
- B (pH = 7) → neutral
- C (pH = 13) → strong base
- D (pH = 2) → strong acid
Consider the following reactions :
(a) Dilute hydrochloric acid reacts with sodium hydroxide.
(b) Magnesium oxide reacts with dilute hydrochloric acid.
(c) Carbon dioxide reacts with sodium hydroxide.
It is found that in each case :
options
- Salt and water is formed.
- Neutral salts are formed.
- Hydrogen gas is formed.
- Acidic salts are formed.
Answer
Salt and water is formed.
Reason — As,
(a) HCl + NaOH → NaCl + H2O — salt and water.
(b) MgO + 2HCl → MgCl2 + H2O — salt and water.
(c) CO2 + 2NaOH → Na2CO3 + H2O — salt and water.
So in each case a salt and water are formed.
Reaction between two elements A and B, forms a compound C. A loses electrons and B gains electrons. Which one of the following properties will not be shown by compound C?
options
- It has high melting point.
- It is highly soluble in water.
- It has weak electrostatic forces of attraction between its oppositely charged ions.
- It conducts electricity in its molten state or aqueous solution
Answer
It has weak electrostatic forces of attraction between its oppositely charged ions.
Reason — When element A loses electrons and element B gains electrons, the compound C formed is ionic in nature. Ionic compounds are held together by strong electrostatic forces of attraction between oppositely charged ions and because of these strong forces, such compounds have high melting points, are often soluble in water and conduct electricity in the molten state or in aqueous solution.
Therefore, the property that will not be shown by compound C is having weak electrostatic forces of attraction between its ions.
The metals obtained from their molten chlorides by the process of electrolytic reduction are :
options
- Gold and silver
- Calcium and magnesium
- Aluminium and silver
- Sodium and iron
Answer
Calcium and magnesium
Reason — Metals like calcium and magnesium are highly reactive and are obtained by the electrolytic reduction of their molten chlorides (CaCl2 and MgCl2).
During electrolysis, the metal ions are reduced at the cathode to form the pure metal and less reactive metals like gold, silver or iron are extracted by other methods and not by electrolysis of molten salts.
Hence, the metals obtained from their molten chlorides by the process of electrolytic reduction are calcium and magnesium.
The formation of magnesium oxide is correctly shown in option :
options
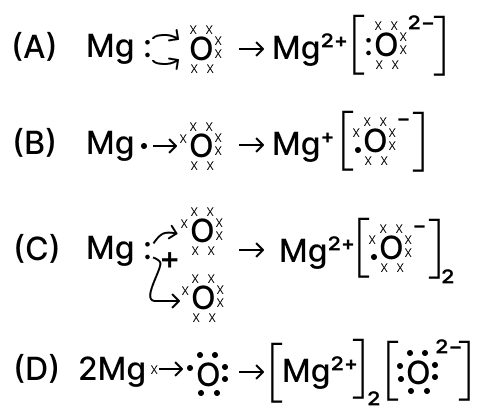
Answer
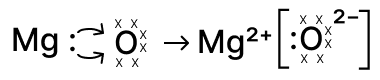
Reason — When magnesium reacts with oxygen each Mg atom loses its two valence electrons to form Mg2+ and an oxygen atom (which has six valence electrons) gains those two electrons to complete its octet and form O2-.
The correct electron-dot representation shows both valence electrons of Mg being transferred to oxygen, leaving Mg2+ (no valence dots) and the oxide ion O2- with eight electrons as shown below :
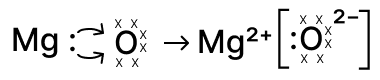
Secretion of less saliva in mouth will effect the conversion of :
options
- Proteins into amino acids
- Fats into fatty acids and glycerol
- Starch into simple sugars
- Sugars into alcohol
Answer
Starch into simple sugars
Reason — Saliva contains the enzyme salivary amylase which begins the digestion of starch in the mouth by breaking it into simpler sugars (like maltose). If less saliva is secreted, there will be reduced salivary amylase activity and therefore decreased conversion of starch into simple sugars.
The plant hormone whose concentration stimulates the cells to grow longer on the side of the shoot which is away from light is :
options
- Cytokinins
- Gibberellins
- Adrenaline
- Auxins
Answer
Auxins
Reason — The plant hormone auxin controls the growth of shoots by stimulating cell elongation. When light falls on one side of a plant shoot, auxin accumulates on the side away from the light, causing the cells there to grow longer and this unequal growth bends the shoot toward the light.
The correct/true statement(s) for a bisexual flower is/are :
(a) They possess both stamen and pistil.
(b) They possess either stamen or pistil.
(c) They exhibit either self-pollination or cross-pollination.
(d) They cannot produce fruits on their own.
options
- (a) only
- (d) only
- (a) and (c)
- (a) and (d)
Answer
(a) and (c)
Reason — A bisexual flower has both stamen (male part) and pistil (female part) present in the same flower, which allows it to undergo self-pollination as well as cross-pollination. Therefore, statements (a) and (c) are true.
If pea plants with round and green seeds (RRyy) are crossed with pea plants having wrinkled and yellow seeds (rrYY), the seeds developed by the plants of F1 generation will be :
options
- 50% round and green
- 75% wrinkled and green
- 100% round and yellow
- 75% wrinkled and yellow
Answer
100% round and yellow
Reason — Crossing RRyy × rrYY gives all F1 offspring genotype RrYy and since R (round) and Y (yellow) are dominant then all F1 seeds will be round and yellow (100%).
The breakdown of glucose has taken the following pathway :
The sites ‘a’ and ‘b’ respectively are :
options
- Mitochondria and Oxygen deficient muscle cells
- Cytoplasm and Oxygen rich muscle cells
- Cytoplasm and Yeast cells
- Cytoplasm and Oxygen deficient muscle cells
Answer
Cytoplasm and Oxygen deficient muscle cells
Reason — Glycolysis (Glucose → Pyruvate + energy) takes place in the cytoplasm of the cell and when oxygen is scarce, pyruvate is converted to lactic acid in oxygen-deficient (anaerobic) muscle cells, releasing a small amount of energy.
Hence the sites for (a) and (b) are cytoplasm and oxygen-deficient muscle cells respectively.
Mirror ‘X’ is used to concentrate sunlight in solar furnace and Mirror ‘Y’ is fitted on the side of the vehicle to see the traffic behind the driver.
Which of the following statements are true for the two mirrors ?
(a) The image formed by mirror ‘X’ is real, diminished and at its focus.
(b) The image formed by mirror ‘Y’ is virtual, diminished and erect.
(c) The image formed by mirror ‘X’ is virtual, diminished and erect.
(d) The image formed by mirror ‘Y’ is real, diminished and at its focus.
options
- (a) and (b)
- (b) and (c)
- (c) and (d)
- (a) and (d)
Answer
(a) and (b)
Reason — Mirror ‘X’ used in a solar furnace is a concave mirror, which concentrates sunlight at its focus, forming a real and diminished image — so statement (a) is true.
Mirror ‘Y’ used as a rear-view mirror is a convex mirror, which forms a virtual, diminished and erect image — so statement (b) is true.
Therefore, the correct statements are (a) and (b).
An old person is suffering from an eye defect caused by weakening of ciliary muscles and diminishing flexibility of the eye lens. If the defect of vision is ‘a’ which can be corrected by lens ‘b’, then ‘a’ and ‘b’ respectively are :
options
- Hypermetropia and convex lens
- Presbyopia and bifocal lens
- Myopia and concave lens
- Myopia and bifocal lens
Answer
Presbyopia and bifocal lens
Reason — As people age the ciliary muscles weaken and the eye lens loses flexibility, so the eye cannot focus on near objects — a condition called presbyopia.
This is typically corrected using bifocal lenses, which provide a separate near-vision segment (convex part) for reading and a distance-vision segment, allowing the person to see both near and far objects comfortably.
Hence the correct pair is presbyopia and bifocal lens.
Which of the following groups do not constitute a food chain ?
(a) Wolf, rabbit, grass, lion
(b) Plankton, man, grasshopper, fish
(c) Hawk, grass, snake, grasshopper, frog
(d) Grass, snake, wolf, tiger
options
- (a) and (d)
- (a) and (c)
- (b) and (c)
- (b) and (d)
Answer
(b) and (d)
Reason — Groups (b) and (d) do not form a food chain because :
(b) Plankton, man, grasshopper, fish — these organisms cannot be placed in a single linear feeding sequence since grasshopper do not feed on plankton and neither fish feeds on grasshopper so the order is ecologically inconsistent.
(d) Grass, snake, wolf, tiger — snakes do not eat grass and wolves/tigers are both top predators (they do not form a single linear chain with grass).
By contrast, the organisms in (a) and (c) can be arranged into plausible feeding sequences for example, grass → rabbit → wolf → lion in (a) and grass → grasshopper → frog → snake → hawk in (c).
The percentage of solar energy which is not converted into food energy by the leaves of green plants in a terrestrial ecosystem is about :
options
- 1%
- 10%
- 90%
- 99%
Answer
99%
Reason — In a terrestrial ecosystem, only about 1% of the solar energy that reaches the leaves is converted into food energy through photosynthesis. This means that nearly 99% of the solar energy is not converted into food energy by green plants.
Assertion (A) : Decomposition reactions are generally endothermic reactions.
Reason (R) : Decomposition of organic matter into compost is an exothermic process.
options
- (A) and (R) are true and (R) is the reason for (A).
- (A) and (R) are true and (R) is not the reason for (A).
- (A) is true, but (R) is false.
- (A) is false, but (R) is true.
Answer
(A) and (R) are true and (R) is not the reason for (A).
Reason —
Assertion (A) is true because most decomposition reactions require an external supply of energy—such as heat, light, or electricity—to break down a compound into simpler substances. In these reactions, the chemical bonds within the compound absorb energy to split apart. For example, when calcium carbonate is heated, it decomposes into calcium oxide and carbon dioxide. Similarly, in the electrolysis of water, electrical energy is needed to decompose water into hydrogen and oxygen gases. Thus, decomposition reactions are generally endothermic in nature.
Reason (R) is true because the decomposition of organic matter into compost involves the action of microorganisms such as bacteria and fungi, which break down complex organic substances like plant and animal waste into simpler compounds. During this biological breakdown, a large amount of heat energy is released into the surroundings. That is why compost heaps often feel warm inside.
Hence, (A) and (R) are true and (R) is not the reason for (A).
Assertion (A) : A human child bears all the basic features of human beings.
Reason (R) : It looks exactly like its parents, showing very little variations.
options
- (A) and (R) are true and (R) is the reason for (A).
- (A) and (R) are true and (R) is not the reason for (A).
- (A) is true, but (R) is false.
- (A) is false, but (R) is true.
Answer
(A) is true, but (R) is false.
Reason —
Assertion (A) is true because every human child inherits the essential characteristics of the human species. These include having two eyes, two legs, a nose, a mouth and the ability to walk upright. Such basic features are determined by the genetic information passed from parents to offspring, ensuring that the child belongs to the same species—Homo sapiens—and displays all fundamental human traits.
Reason (R) is false because although a child resembles its parents, it never looks exactly like them due to genetic variations that occur during sexual reproduction, where genes from both parents combine in unique ways. As a result, the child inherits some features from each parent but also shows differences, making every human being genetically and physically distinct.
Hence, (A) is true, but (R) is false.
Assertion (A) : No two magnetic field lines are found to cross each other.
Reason (R) : The compass needle cannot point towards two directions at the point of intersection of two magnetic field lines.
options
- (A) and (R) are true and (R) is the reason for (A).
- (A) and (R) are true and (R) is not the reason for (A).
- (A) is true, but (R) is false.
- (A) is false, but (R) is true.
Answer
(A) and (R) are true and (R) is the reason for (A).
Reason —
Assertion (A) is true because magnetic field lines represent the direction of the magnetic field at different points in space. If two field lines were to cross, it would mean that the magnetic field has two different directions at the same point, which is not possible in nature. Therefore, magnetic field lines never intersect each other.
Reason (R) is true because a compass needle always aligns itself along the direction of the magnetic field at a point and if two magnetic field lines were to intersect, the compass needle would have to point in two directions simultaneously, which is impossible.
Hence, (A) and (R) are true and (R) is the reason for (A).
Assertion (A) : The amount of ozone in the atmosphere began to drop sharply in the 1980s.
Reason (R) : The oxygen atoms combine with molecular oxygen to form ozone.
options
- (A) and (R) are true and (R) is the reason for (A).
- (A) and (R) are true and (R) is not the reason for (A).
- (A) is true, but (R) is false.
- (A) is false, but (R) is true.
Answer
(A) and (R) are true and (R) is not the reason for (A).
Reason —
Assertion (A) is true because scientific studies in the 1980s showed a significant depletion of the ozone layer, particularly over Antarctica due to the release of chlorofluorocarbons (CFCs) and other pollutants, which break down ozone molecules in the stratosphere, leading to the formation of the ozone hole.
Reason (R) is true because it describes how ozone is naturally formed in the atmosphere (O + O2 → O3). However, it does not explain why the ozone amount dropped in the 1980s. The drop was due to ozone destruction by CFCs, not its natural formation process.
Hence, (A) and (R) are true and (R) is not the reason for (A).
A student performs the following experiment in his school laboratory.

List two observations to justify that in this experiment a chemical change has taken place.
Answer
When zinc granules react with dilute sulphuric acid, a chemical change takes place.
Two observations that justify this are :
- Effervescence (bubbling) is observed, indicating that a gas (hydrogen) is being evolved.
- The zinc granules gradually dissolve / become smaller and the flask becomes warm, showing that a new substance is formed and heat is produced.
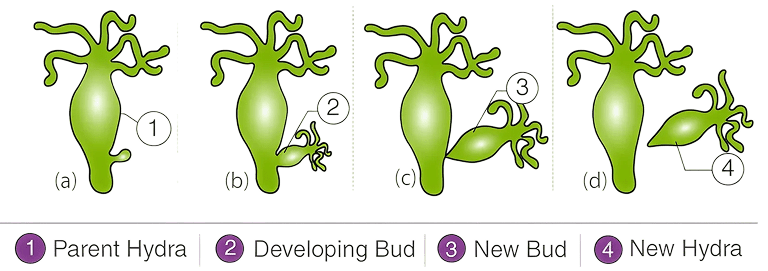
Draw labelled diagrams to show different stages of budding in Hydra.
Answer
The labelled diagram to show different stages of budding in Hydra is given below :
Besides minimising the loss of blood, why is it essential to plug any leak in a blood vessel? Name the component of blood which helps in this process and state how this component perform this function.
Answer
It is essential to plug any leak in a blood vessel not only to minimise the loss of blood but also to prevent the entry of germs into the body, which could cause infection. The component of blood that helps in this process is platelets. When a blood vessel is injured, platelets collect and stick at the site of injury, releasing chemicals that help in the formation of a blood clot, which seals the wound and stops bleeding.
(a) The transport system in plants is relatively slower than in animals. Give reasons.
(b) State the role of phloem in the transport of materials in plants.
Answer
(a) The transport system in plants is slower because they do not have a pumping organ like the heart. Movement of water and minerals occurs mainly due to transpiration pull and root pressure, and food moves by pressure gradients in phloem. These processes are slower than the rapid blood circulation in animals.
(b) The phloem transports food materials (mainly sucrose) produced in the leaves during photosynthesis to all other parts of the plant and this movement of food from leaves to storage organs or growing regions is called translocation.
An object is placed at a distance of 60 cm from a concave lens of focal length 30 cm. Use lens formula to find the position of the image formed in this case.
Answer
Given,
- Object distance () = -60 cm
- Focal length of the lens () = -30 cm
Here, the negative sign for shows that the object is placed in front of the lens, and the negative sign for shows that the lens is concave (diverging).
By, using the lens formula,
Hence, the image is formed in front of the lens at a distance of 20 cm from it.
A wire of resistance R is cut into three equal parts. If these three parts are then joined in parallel, calculate the total resistance of the combination so formed.
Answer
Given,
- Resistance of the wire = ()
- Number of parts cut of the wire = 3
As, resistance of a wire is directly proportional to its length then on increasing the length resistance increases and vice versa.
As all part are of equal length then resistance of each part is given by,
When three parts are connected in parallel combination then the total resistance is given by,
Hence, the total resistance of the combination so formed .
Define electric power. When do we say that the power consumed in an electric circuit is 1 watt ?
Answer
Electric power is defined as the rate at which electrical energy is consumed or converted into other forms of energy in an electric circuit.
Mathematically, electric power is given as :
P = V × I
where
- P is power,
- V is potential difference, and
- I is current
On putting, V = 1 V and I = 1 A
P = 1 x 1 = 1 W
So, the power consumed in a circuit is said to be 1 watt when 1 ampere of current flows through a conductor having a potential difference of 1 volt across its ends.
"Excessive use of chemicals and pesticides in agriculture adversely effect the environment." Justify this statement.
Answer
Excessive use of chemicals and pesticides in agriculture harms the environment in several ways :
- These substances not only kill harmful pests but also destroy useful soil organisms that maintain soil fertility.
- The chemical residues often seep into the soil and mix with groundwater, polluting water sources and affecting aquatic life.
- Many of these chemicals are non-biodegradable and enter the food chain, where they get magnified at higher trophic levels (biomagnification), causing serious health problems in animals and humans.
- Prolonged use of such chemicals degrades soil quality and disturbs the ecological balance of the ecosystem.
(a) "Displacement reactions also play a key role in extracting metals in the middle of the reactivity series." Justify this statement with two examples.
(b) Why can metals high up in the reactivity series not be obtained by reduction of their oxides by carbon ?
Answer
(a) Metals in the middle of the reactivity series are usually obtained from their compounds using displacement reactions, where a more reactive element displaces a less reactive metal from its compound. For example:
Iron is obtained from its oxide using carbon monoxide as the reducing agent:
Fe2O3 + 3CO → 2Fe + 3CO2Zinc oxide is reduced by carbon to form zinc metal:
ZnO + C → Zn + CO
(b) Metals high up in the reactivity series such as sodium, potassium, calcium, magnesium and aluminium cannot be obtained by reducing their oxides with carbon because they have a strong affinity for oxygen. Their oxides are very stable and carbon is not reactive enough to displace these metals. Therefore, such metals are extracted by electrolysis of their molten compounds.
With the help of an activity, explain the conditions under which iron articles get rusted.
Answer
To show the conditions under which iron gets rusted, take three test tubes containing iron nails and set them up as follows:
- Test tube 1: Contains distilled water — both air (oxygen) and water are present.
- Test tube 2: Contains boiled water with a layer of oil — only water is present, no air.
- Test tube 3: Contains anhydrous calcium chloride — only dry air is present, no water.
After a few days, only the iron nails in test tube 1 get rusted, showing that both air (oxygen) and moisture (water) are necessary for rusting. Thus, rusting of iron occurs only when iron, oxygen, and water are all present.
(a) Name two metals which react violently with cold water. List any three observations which a student notes when these metal are dropped in a beaker containing water.
(b) Write a test to identify the gas evolved (if any) during the reaction of these metals with water.
Answer
(a) The two metals which react violently with cold water are sodium (Na) and potassium (K).
Three observations when these metals are dropped in water are:
- Fizzing and hissing due to rapid evolution of hydrogen gas.
- The metal piece moves rapidly on the water surface and melts.
- The reaction produces heat, making the water alkaline due to formation of metal hydroxide.
(b) The gas evolved is hydrogen which can be tested by bringing a burning matchstick near the mouth of the test tube.The gas burns with a ‘pop’ sound, confirming the presence of hydrogen.
Plants have neither a nervous system nor muscles, even then they respond to stimuli. For example, the leaves of chhui-mui (touch-me-not) plant when touched begin to fold up and droop.
(a) How is the information communicated in "touch-me-not" plants ?
(b) What enables the plant cells to bring out the observable response ?
(c) Differentiate the movement mentioned above from the movement of tendrils in a pea plant.
Answer
(a) In the touch-me-not (chhui-mui) plant, the information about touch is communicated through electrical and chemical signals from the affected part to the nearby cells. These signals cause changes in the water pressure inside cells of certain specialized cells at the base of the leaflets and petioles.
(b) The change in water pressure enables the plant cells to bring about the observable response. When touched, water moves out of the cells, making them flaccid, which causes the leaves to fold up and droop.
(c) The movement in the touch-me-not plant is a nastic movement, which occurs independently of the direction of the stimulus (the leaves fold whether touched from any side). In contrast, the movement of tendrils in pea plants is a tropic movement, which occurs in the direction of the stimulus (tendrils bend towards or coil around the object they touch).
(a) What are chromosomes ?
(b) Explain in brief how stability of DNA content of a species is ensured in sexually reproducing organisms ?
Answer
(a) Chromosomes are thread-like structures present in the nucleus, made up of DNA and proteins. They carry genes and hence transmit genetic information from one generation to the next.
(b) In sexually reproducing organisms, meiosis produces haploid gametes with half the number of chromosomes. During fertilisation, the male and female gametes fuse to form a diploid zygote, restoring the original chromosome number. This alternate halving (meiosis) and doubling (fertilisation) ensures that the DNA content of the species remains stable from generation to generation.
Draw ray diagrams to show the nature, position and relative size of the image formed by a convex mirror when the object is placed
(a) at infinity and
(b) between infinity and pole P of the mirror.
Answer
The ray diagrams to show the nature, position and relative size of the image formed by a convex mirror is given below :
(a) When object is at infinity :
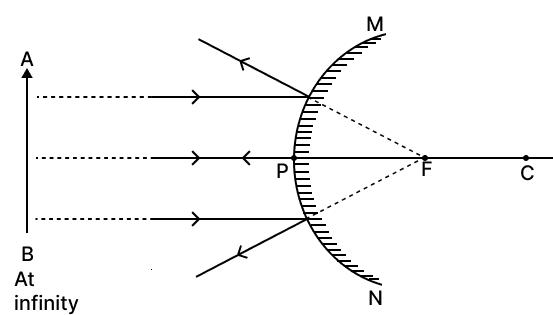
(b) When the object is between infinity and pole P of the mirror :

Consider the following electric circuit :

Calculate the values of the following :
(a) The total resistance of the circuit
(b) The total current drawn from the source
(c) Potential difference across the parallel combination of 10 Ω and 15 Ω resistors
Answer
(a) Given,
- Battery voltage () = 15 V
From the figure,
10 Ω and 15 Ω resistors are in parallel then their total resistance is given by,
Again, 40 Ω and 60 Ω are also in parallel then their total resistances given by,
Now, and are in series then total resistance of the circuit is given by,
Hence, the total resistance of the circuit is 30 Ω.
(b) Current drawn from the battery is given by,
Hence, the total current drawn from the source is 0.5 A.
(c) Potential difference across the parallel combination of 10 Ω and 15 Ω resistors is given by,
Hence, the potential difference across the parallel combination of 10 Ω and 15 Ω resistors is 3 V.
(a) Write the relationship between resistivity and resistance of a cylindrical conductor of length l and area of cross-section A. Hence derive the SI unit of resistivity.
(b) Why are alloys used in electrical heating devices?
Answer
(a) Resistance of a cylindrical conductor is given by,
Then,
Hence, SI unit of ρ is Ω.m.
(b) Alloys are used in electrical heating devices because :
- they have relatively high resistivity, so they produce a large amount of heat for a given current and voltage;
- they have high melting points, so they do not melt at the high temperatures reached during heating;
- they are resistant to oxidation and corrosion at high temperatures, so the heating element lasts longer;
- their mechanical strength and ductility are suitable for making durable coils.
Pure metals (like copper) have low resistivity and oxidise or melt easily, so they are unsuited for heating elements.
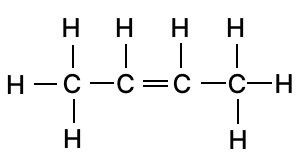
(a) Draw two isomeric structures of Butene (C4H8)
(b) Name the following compounds :

(c) Write the chemical equations for the following reactions. Mention one essential condition each for these reactions to take place.
- Ethanol undergoes complete oxidation
- Propene undergoes hydrogenation
- Ethanoic acid reacts with ethanol
Answer
(a) Butene (C4H8) has two possible structural isomers due to the position of the double bond:
- 1-butene

- 2-butene
(b)
1-chloropropane
butan-2-one
(c)
1. Complete oxidation of ethanol :
Essential condition : Sufficient (excess) oxygen and ignition/heat so combustion proceeds to CO2 and H2O.
2. Hydrogenation of propene :
Essential condition : Presence of hydrogen and a hydrogenation catalyst (e.g. nickel, platinum or palladium) — often with mild heating/pressure.
3. Ethanoic acid reacts with ethanol (esterification) :
Essential condition : Acid catalyst (concentrated H2SO4) and gentle heating (reflux) to form ethyl ethanoate; removal of water drives the equilibrium to the right.
(a) A carbon compound X is a good solvent. On reaction with sodium, X forms two products Y and Z. Z is used to convert vegetable oil into vegetable ghee. Identify and name X, Y and Z. Also write the equation of reaction of X with sodium to justify your answer.
(b) Write chemical equation to show what happens when ethanol :
- burns in oxygen/air.
- is heated at 443 K in excess conc. H2SO4.
- reacts with acidified potassium dichromate.
Answer
(a)
- X = Ethanol (C2H5OH) — a good solvent.
- Y = Sodium ethoxide (C2H5ONa).
- Z = Hydrogen gas (H2) — hydrogen is used for hydrogenation to convert vegetable oil into vegetable ghee.
Reaction with sodium :
(b)
1. Combustion of ethanol : Ethanol burns in air to form carbon dioxide and water, releasing heat and light which shows ethanol acts as a clean fuel.
Chemical equation :
2. Dehydration of ethanol (443 K, conc. H2SO4) : When ethanol is heated with concentrated sulphuric acid, it loses a water molecule and forms ethene, an unsaturated hydrocarbon.
Chemical equation :
3. Oxidation by acidified potassium dichromate : Ethanol is oxidised to ethanoic acid by acidified potassium dichromate, with the solution’s colour changing from orange to green.
Chemical equation :
(a) Write the functions of the following parts of human female reproductive system :
- Ovary
- Fallopian tube
- Uterus
(b) State briefly two contraceptive methods used by human males.
Answer
(a) The functions of the following parts of human female reproductive system are :
- Ovary : The ovaries produce female gametes (ova or eggs) and secrete female sex hormones like estrogen and progesterone.
- Fallopian tube : It receives the ovum released by the ovary and is the site where fertilisation of the egg by sperm usually occurs.
- Uterus : The fertilised egg implants in the uterine wall and develops into an embryo and it provides nourishment and protection to the developing foetus.
(b) Two contraceptive methods used by human males are :
- Use of condoms: A barrier method that prevents sperm from entering the female reproductive tract.
- Vasectomy: A surgical method in which the vas deferens are cut and tied to prevent the release of sperm during ejaculation.
(a) Differentiate between self-pollination and cross-pollination.
(b) Identify A, B and C in the diagram given below and write one function of each.

Answer
(a)
| Self-pollination | Cross-pollination |
|---|---|
| Transfer of pollen grains from the anther to the stigma of the same flower or another flower on the same plant. | Transfer of pollen grains from the anther of one flower to the stigma of a flower on another plant of the same species. |
| No genetic variation; offspring are identical to parent. | Produces genetic variation; offspring show new traits. |
| No external agents are needed. | Requires agents like wind, water, or insects. |
(b) Identification and functions of parts (A, B, and C) :
A – Stigma
Function : It receives pollen grains during pollination.B – Pollen tube
Function : It carries male gametes (sperm nuclei) from pollen grain to the ovule for fertilisation.C – Female gamete (egg or ovum)
Function : It fuses with the male gamete during fertilisation to form the zygote, which develops into a seed.
(a) The power of a lens 'X' is -2·5 D. Name the lens and determine its focal length in cm. For which eye defect of vision will an optician prescribe this type of lens as a corrective lens ?
(b) "The value of magnification 'm' for a lens is -2." Using new Cartesian Sign Convention and considering that an object is placed at a distance of 20 cm from the optical centre of this lens, state :
- the nature of the image formed;
- size of the image compared to the size of the object;
- position of the image, and
- sign of the height of the image.
(c) The numerical values of the focal lengths of two lenses A and B are 10 cm and 20 cm respectively. Which one of the two will show higher degree of convergence/divergence? Give reason to justify your answer.
Answer
(a) Given,
- Power of the lens () = -2.5 D
As power of the lens is negative it means the lens is concave (diverging).
Power of a lens is given by,
Where is the focal length of the lens in metre.
Since the lens is diverging in nature then such a lens is prescribed to correct myopia (short-sightedness).
(b) Given,
- Magnification () = -2
- Object distance () = -20 cm
Since m is negative it means image is real and inverted.
Since m = 2 so it means size of the image is two times the size of the object.
Let, the position of the image be .
As, magnification of an object is given by,
- As, < 0 it means image is inverted which implies sign of the height of the image negative.
(c) Given,
- Focal length of lens A = 10 cm
- Focal length of lens B = 20 cm
As, power of a lens is given by,
,
Hence, lens A will show a higher degree of convergence/divergence because it has a smaller focal length and therefore higher power.
(a) Draw a ray diagram to show the refraction of a ray of light through a rectangular glass slab when it falls obliquely from air into glass.
(b) State Snell’s law of refraction of light.
(c) Differentiate between the virtual images formed by a convex lens and a concave lens on the basis of :
- object distance, and
- magnification
Answer
(a) The ray diagram showing the refraction of a ray of light through a rectangular glass slab when it falls obliquely from air into glass is given below :
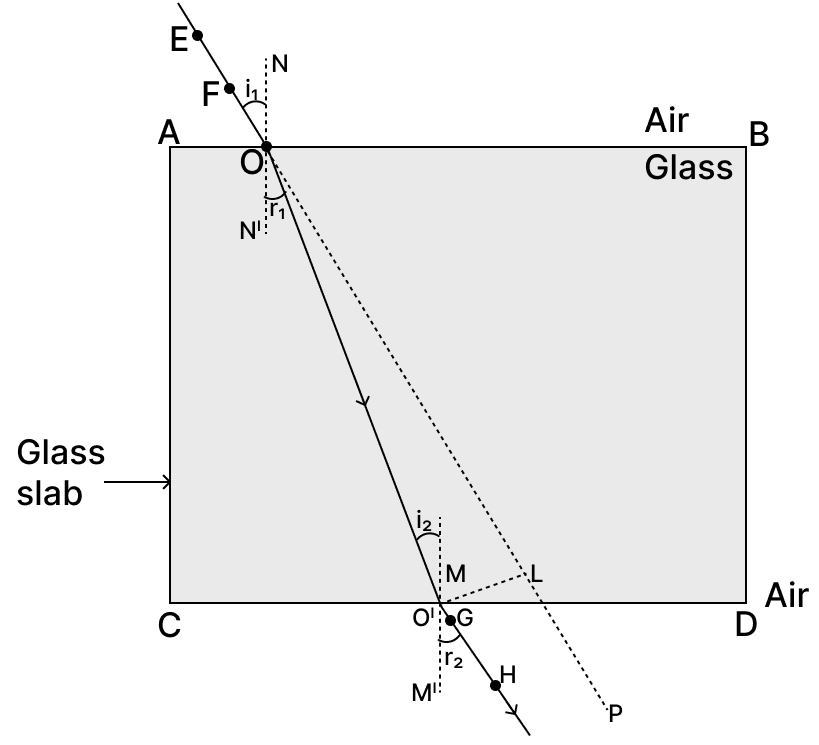
(b) Snell's law states that the ratio of the sine of the angle of incidence (i) to the sine of the angle of refraction (r) is a constant for a given pair of media i.e.,
where n21 is the refractive index of the second medium with respect to the first.
(c)
| Basis | Convex Lens | Concave Lens |
|---|---|---|
| 1. Object distance | A virtual image is formed when the object is placed between the optical centre and the focus (F). | A virtual image is formed for all positions of the object. |
| 2. Magnification | The image is virtual, erect, and magnified. | The image is virtual, erect, and diminished. |
Seawater contains many salts dissolved in it. Common salt is separated from these salts. Deposits of solid salt are also found in several parts of the world. These large crystals are often brown due to impurities. This is called rock salt and is mined like coal. The common salt is an important raw material for chemicals of daily use.
(a) Write balanced chemical equations to show the products formed during electrolysis of brine.
(b) List two uses of any one product obtained during electrolysis of brine.
(c) A mild non-corrosive basic salt ‘A’, used for faster cooking, is strongly heated to produce a compound ‘B’, that is used for removing permanent hardness of water. Identify A and B and also write the equation for the reaction that occurs when A is heated.
OR
(c) Define water of crystallisation. Give two examples of salts that have water of crystallisation.
Answer
(a) Electrolysis of brine (aqueous NaCl) gives chlorine at anode, hydrogen at cathode and sodium hydroxide in solution:
2NaCl + 2H2O ⟶ 2NaOH + H2(↑) + Cl2(↑)
(b) Two uses of chlorine :
- Disinfecting and purifying drinking water.
- Manufacture of bleaching agents and many organic chemicals (e.g. PVC).
(c)
- A = sodium hydrogen carbonate (sodium bicarbonate, NaHCO3).
- B = sodium carbonate (Na2CO3), used to remove permanent hardness of water.
On strong heating of A (NaHCO3) :
OR
(c) Water of crystallisation is the definite number of water molecules chemically incorporated in one formula unit of a crystalline salt.
Examples :
- Copper(II) sulfate pentahydrate (CuSO4⋅5H2O)
- Sodium carbonate decahydrate, Na2CO3⋅10H2O.
The maintenance functions of all living organisms must go on even when they are not doing anything particular. Even when we are just sitting in a class or even asleep, this maintenance job has to go on. These maintenance processes require energy to prevent damage and break-down of cells and tissues, which is obtained by the individual organism from the food prepared by the autotrophs, called producers.
(a) Name and define the process by which green plants prepare food.
(b) Write chemical equation involved in the above process.
(c) State in proper sequence the events that occur in synthesis of food by desert plants.
OR
(c) Explain giving reasons what happens to the rate at which the green plants will prepare food
- during cloudy weather, and
- when stomata get blocked due to dust.
Answer
(a) Photosynthesis is the process by which green plants use carbon dioxide and water, in the presence of sunlight and chlorophyll, to make food (glucose) and release oxygen.
(b) The chemical equation for photosynthesis is:
(c) In desert plants (like cacti), photosynthesis occurs in a slightly modified way :
- Stomata open at night to take in carbon dioxide (to prevent water loss).
- The absorbed CO2 is stored as an intermediate compound.
- During the day, when sunlight is available, this stored CO2 is used for photosynthesis while stomata remain closed.
OR
- During cloudy weather : The rate of photosynthesis decreases because less sunlight reaches the plants, reducing the energy available for the process.
- When stomata get blocked due to dust : The entry of CO2 into leaves is reduced, slowing down photosynthesis since CO2 is one of the main raw materials.
In our homes, we receive the supply of electric power through a main supply also called mains, either supported through overhead electric poles or by underground cables. In our country the potential difference between the two wires (live wire and neutral wire) of this supply is 220 V.
(a) Write the colours of the insulation covers of the line wires through which supply comes to our homes.
(b) What should be the current rating of the electric circuit (220 V) so that an electric iron of 1 kW power rating can be operated ?
(c) What is the function of the earth wire? State the advantage of the earth wire in domestic electric appliances such as electric iron.
OR
(c) List two precautions to be taken to avoid electrical accidents. State how these precautions prevent possible damage to the circuit/appliance.
Answer
(a) The insulation colours of the wires in domestic circuits are:
- Live wire : Red
- Neutral wire : Black
- Earth wire : Green
(b) Given,
- Power rating of the electric iron () = 1 kW = 1000 W
- Supply voltage () = 220 V
Current rating of the iron is given by,
Hence, the current rating of the circuit should be about 4.5 A.
(c) The earth wire provides a path for the leakage current to flow safely into the ground in case of insulation failure.
Advantage : It prevents the metal body of appliances like an electric iron from becoming live, thereby protecting users from electric shocks.
OR
(c) Two precautions to avoid electrical accidents:
- Use of proper fuse: Prevents excessive current from flowing through the circuit by melting and breaking the circuit, protecting appliances from damage.
- Never touch electrical appliances with wet hands: Water conducts electricity and can cause electric shocks; keeping hands dry ensures safety.