Which one is a kind of matter?
- Light
- Petroleum
- Sound
- Heat
Answer
Petroleum
Reason — Matter is anything that has mass and occupies space. Petroleum is considered as a matter because it is a liquid substance that has mass and occupies space. Whereas, light, heat and sound has no definite mass and do not occupy space.
All kinds of matter:
- occupy space and have a definite mass
- have mass and a definite shape
- can change their states
- have a definite volume
Answer
occupy space and have a definite mass
Reason — All kind of matter occupy space and have a definite mass. However, only solids have definite shape, not all forms of matter can change their states and only solids and liquids have definite volume. Hence, other options are not correct.
The space occupied by matter is called :
- mass
- area
- volume
- weight
Answer
volume
Reason — The space occupied by matter is called its volume. The greater the size of an object, the larger will be the space occupied by it.
The quantity of matter in an object is called as:
- mass
- volume
- density
- weight
Answer
mass
Reason — The quantity or amount of matter in an object is called its mass. The larger an object, the greater will be its mass.
Which of the following is a kind of man-made matter?
- Coal
- Cotton
- Plastic
- Silk
Answer
Plastic
Reason — Plastic is a non-living man-made or artificial matter. It is produced artificially from natural matter. Whereas, coal, cotton and silk are natural matters.
An object which is not made up of wood is:
- Desk
- Soap
- Chair
- Handle
Answer
Soap
Reason — Soap is made of fats or vegetable oil and alkali. Desk, chair and handle can be made from wood.
Select natural and man-made matter from the following list:
Wood, plastic, silk, medicines, detergents, coal, water, ceramic, cotton, glass, nylon, fruits.
Answer
Natural Matter — Wood, silk, coal, water, cotton, fruits
Man-made Matter — Plastic, medicines, detergent, ceramic, glass, nylon
Define matter.
Answer
"Anything that has mass and occupies space is called matter".
What are the two main types of matter? Give two examples for each type.
Answer
Two main types of matter :
- Living matter — Matter that shows characteristics of life like growth, reproduction, response to stimuli, etc.,
Examples : Humans, trees, etc. - Non-living matter — Matter that does not show characteristics of life. They do not grow, move or reproduce on their own.
Examples : Plastics, cotton, etc.
Why are plants and animals considered as matter?
Answer
Plants and animals are living matter as they have mass and they occupy space. They can grow, move and reproduce on their own and are also made up of atoms and molecules. So, they are considered as matter.
The state of matter which has no definite shape or volume is called:
- solid
- liquid
- gas
- none of these
Answer
gas
Reason — Gas is state of matter that has neither a definite shape nor a definite volume. It can be compressed inside a small container and can also spread into a large space area.
The largest intermolecular gaps occur in:
- water
- iron ball
- common salt
- air
Answer
air
Reason — In case of gases, the molecules hardly attract each other. They lie far apart from each other and hence, the intermolecular spaces are very large.
A solid:
- has a definite shape
- cannot flow
- cannot be compressed easily
- all of the above
Answer
all of the above
Reason — In solids, molecules are closely packed with very less intermolecular space. This makes solids hard and difficult to compress, giving them a fixed shape and size. So, they cannot flow like liquids.
Which of the following diffuses very fast?
- Water
- Gold
- Milk
- Nitrogen
Answer
Nitrogen
Reason — Nitrogen is a gas. Gases diffuse very fast because the particles of gases have enough space between them which allows them to move freely and to mix up easily.
Which of the following shows the strongest cohesive force ?
- Petrol
- Oxygen
- Mercury
- Hydrogen
Answer
Mercury
Reason — The force of attraction between like particles or molecules is called Cohesive force. Mercury, although a liquid, shows a strong cohesive force. Whereas, oxygen and hydrogen are gases and have least cohesive force. Petrol have a cohesive force less than mercury.
Classify the following into solids, liquids and gases.
Oxygen, milk, common salt, wax, stone, L.P.G., carbon dioxide, sugar, mercury, coal, blood, butter, copper, coconut oil, kerosene.
Answer
- Solids — Common salt, wax, stone, sugar, coal, butter, copper
- Liquids — Milk, mercury, blood, coconut oil, kerosene
- Gases — Oxygen, L.P.G, carbon dioxide
Name the smallest particle from which matter is made of.
Answer
Matter is made up of extremely small particles called atoms.
What are molecules ?
Answer
A molecule is the smallest unit of matter which exhibits all the properties of that kind of matter and is capable of independent existence.
Define:
(a) Intermolecular force of attraction.
(b) Intermolecular space.
(c) Cohesive force
(d) Diffusion
(e) Brownian movement
Answer
(a) Particles of matter are held together by a force of attraction that exists between them. This force is known as intermolecular force of attraction.
(b) Particles of matter have space between them which is called inter-particle or intermolecular space or gap.
(c) The force of attraction between like particles or molecules is called cohesive force. It is this force which holds the molecules of a matter together.
(d) The phenomenon of intermixing of particles of one kind with another kind is called diffusion.
(e) The zig-zag motion of particles suspended in a medium is called Brownian movement. It is named after the scientist Robert Brown.
Give one difference between atoms and molecules.
Answer
| Atoms | Molecules |
|---|---|
| An atom is the smallest possible unit of matter that exhibits all the properties of matter. | Two or more atoms combine together to form a minute particle called molecule. |
| Atoms usually do not have an independent existence. | Molecule is capable of independent existence. |
What are fluids ? Give two examples.
Answer
All substances that can flow are called fluids. Both gases and liquids are fluids.
Examples : Water, oxygen etc.,
Name the three states of matter and define them.
Answer
Solid — A solid has a definite shape and a definite volume. Molecules in the solid state are packed very close to each other.
Liquid — A liquid has a definite volume but no definite shape. The molecules in liquids are less closely packed than solids.
Gas — A gas has neither a definite shape nor a definite volume.
Give reason:
Liquids and gases flow but solids do not.
Answer
In solids, the molecules are closely packed with negligible intermolecular space between them. There is a strong force of attraction between the molecules. This makes them hard so they cannot flow. However, liquids and gases have more intermolecular space between them compared to solids, so they can flow freely.
Give reason:
A gas fills up the space available to it.
Answer
In case of gases, the molecules are hardly attracted to each other with very large intermolecular space. As a result, gases have neither a fixed shape nor a fixed volume. They completely fill up the space available to them.
Give reasons:
The odour of scent spreads in a room.
Answer
When you spray scent in a room, its particles mix with air molecules and diffuse and gradually spread throughout the room.
Give reason:
We can walk through air.
Answer
Air is a gas, and its particles are widely spaced, offering very little resistance to movement. So we can easily walk through it.
Give reason:
Liquids have a definite volume but no definite shape.
Answer
In liquids, the molecules are not very closely packed. They are moderately attracted to each other giving it a definite volume. Thus, the intermolecular spaces are larger and the molecules are able to move freely. This makes a liquid to take shape of the container.
Give reason:
When a teaspoon of sugar is added to half a glass of water and stirred, the water level in the glass remains unchanged.
Answer
This is because sugar particles settle into the spaces between water molecules. This is a result of intermolecular spaces in liquids.
Give reason:
When an empty gas jar is inverted over a gas jar containing a coloured gas, the gas also spreads into the empty jar.
Answer
This is because of diffusion. Gases diffuse very fast they move randomly and mix with each other, even without stirring.
Give reason:
A red ink drop added to a small amount of water in a glass turns the water red in some time.
Answer
This is because of diffusion. Liquid diffuse slower than gas, so when we add drop of red ink to a small amount of water in a glass, it will diffuse slowly into entire glass of water.
Give reason:
Solids usually have higher density than liquids and gases.
Answer
In solids, particles are tightly packed, making them denser than liquids and gases.
Give reason:
An egg is kicked out of a bottle when air is blown inside the bottle.
Answer
Blowing air increases the air pressure inside the bottle. The high pressure pushes the egg outward.
A kind of matter which can sublime is :
- water
- plastic
- milk
- iodine
Answer
iodine
Reason — Iodine is a substance that directly change from from the solid state to gaseous state without passing through the liquid state. Iodine sublimes into a violet gas when heated.
A substance which can change its state is:
- wood
- oxygen
- paper
- cloth
Answer
oxygen
Reason — Oxygen can change from its gaseous state to its liquid state at lower temperature by the process called condensation or liquefaction.
The process by which a solid changes into a liquid is called :
- freezing
- melting
- condensation
- evaporation
Answer
melting
Reason — The process by which a substance changes from solid state to liquid state is called melting or fusion.
The change of state of liquid into vapour below its boiling points is called:
- melting
- freezing
- boiling
- evaporation
Answer
evaporation
Reason — The change of state of a liquid into vapour below boiling point is called evaporation. Evaporation takes place even at room temperature but it becomes faster on heating.
When a substance is heated, it can:
- undergo a chemical change
- change its state
- show expansion
- all of the above
Answer
all of the above
Reason — Heating causes substance to change its physical state as well as bring about a chemical change. All the three states of matter expands on heating, which is referred as thermal expansion. Hence, all the options are correct.
(a) Water is matter because it has ............... and occupies ...............
(b) Any matter which has a definite ............... but no definite shape is called a ................
(c) ............... and ............... can flow.
(d) The molecules are at a greater distance in ............... as compared to liquids.
(e) Water boils at ............... °C.
(f) The physical state of a substance, which has neither fixed volume nor fixed shape is a ...............
Answer
(a) Water is matter because it has mass and occupies space.
(b) Any matter which has a definite volume but no definite shape is called a liquid.
(c) Liquids and gases can flow.
(d) The molecules are at a greater distance in gases as compared to liquids.
(e) Water boils at 100°C.
(f) The physical state of a substance, which has neither fixed volume nor fixed shape is a gas.
Write whether the following statements are true or false.
(a) Only water can exist in three different states. ...............
(b) If the container in which a gas is collected has an opening, the gas will flow out and spread itself indefinitely. ...............
(c) Solids have the largest inter-molecular space. ...............
(d) There is no difference between evaporation and boiling. ...............
(e) All solids, on heating, first change to liquid and then to the gaseous state. ...............
(f) The intermolecular force of attraction is the weakest in gases. ...............
(g) A gas has no free surface. ...............
Answer
(a) False
Correct Statement — Many substances like carbon dioxide, iodine, etc., can exist in solid, liquid, and gaseous states under suitable conditions.
(b) True
(c) False
Correct Statement — Gases have the largest intermolecular space. Solids have very tightly packed particles.
(d) False
Correct Statement — Evaporation is a slow surface-level process that occurs at any temperature, while boiling is a rapid, bulk process that happens at the boiling point.
(e) False
Correct Statement — Some solids like camphor, iodine, and dry ice undergo sublimation, changing directly from solid to gas without becoming liquid.
(f) True
(g) True
Match the following
| Column A | Column B |
|---|---|
| (a) Solids | (i) Can flow in all directions. |
| (b) Sublimation | (ii) The temperature at which a liquid changes into its gaseous state |
| (c) Boiling point | (iii) Can have any number of free surface |
| (d) Gases | (iv) Gaps between molecules |
| (e) Intermolecular space | (v) Change of state directly from solid to gas. |
Answer
| Column A | Column B |
|---|---|
| (a) Solids | (iii) Can have any number of free surface |
| (b) Sublimation | (v) Change of state directly from solid to gas. |
| (c) Boiling point | (ii) The temperature at which a liquid changes into its gaseous state |
| (d) Gases | (i) Can flow in all directions. |
| (e) Intermolecular space | (iv) Gaps between molecules |
Select the odd one out from the following:
(a) solid, liquid, gas, volume
(b) water, fruit juice, air, stone, milk
(c) Iron, aluminium, wood, gold
(d) Freezing, condensation, melting point, evaporation
Answer
(a) Volume
Reason — Solid, liquid and gas are the states of matter, while volume is a property of matter.
(b) Stone
Reason — Water, fruit juice, air and milk are fluids and can flow, while stone is a solid and cannot flow.
(c) Wood
Reason — Iron, aluminium and gold are metals, whereas wood is a non-metal.
(d) Melting point
Reason — Freezing, condensation and evaporation are processes of state change, while melting point is a temperature, not a process.
For each of the following statements, say whether it describes a solid, a liquid or a gas.
(a) Particles move about very quickly but do not leave the surface.
(b) Particles are quite close together.
(c) Particles are far apart and move in all directions.
Answer
(a) Liquid
(b) Solid
(c) Gas
Name the phenomenon which causes the following changes:
(a) Formation of water vapour from water.
(b) Disappearance of camphor when exposed to air.
(c) Conversion of ice into water.
(d) Conversion of water into steam.
Answer
(a) Evaporation
(b) Sublimation
(c) Melting
(d) Boiling or vaporisation
Give two examples for each of the following:-
(a) Substances which sublime.
(b) Substances which do not change their state.
(c) Substances which are rigid and not compressible.
Answer
(a) Camphor and dry ice (solid carbon dioxide)
(b) Paper and sugar
(c) Stone and iron
Define the following terms:
(a) Fusion
(b) Vaporisation
(c) Condensation
(d) Sublimation
(e) Diffusion
(f) Melting point
(g) Boiling point
(h) Liquefaction
(i) Evaporation
Answer
(a) The process by which a substance changes from solid state to liquid state is called melting or fusion.
(b) The process by which a substance changes from a liquid state to vapour state on heating is called vaporisation or boiling.
(c) The process by which a substance in gaseous state changes into its liquid state is called condensation or liquefaction.
(d) The conversion of a solid substance into its vapour without undergoing liquid state on heating is called sublimation.
(e) The phenomenon of intermixing of particles of one kind with another kind is called diffusion.
(f) The fixed temperature at which a solid changes into a liquid at a given pressure is called its melting point.
(g) The fixed temperature at which a liquid starts changing into gaseous state is called its boiling point.
(h) The process by which a substance in gaseous state changes into its liquid state is called condensation or liquefaction.
(i) The change of state of a liquid into vapour below boiling point is called as evaporation.
(a) Define: interconversion of states of matter.
(b) What are the two conditions for the interconversion of states of matter?
Answer
(a) The process by which matter changes from one state to another and back to the original state, without any change in its chemical composition is called interconversion of states of matter.
(b) The two conditions for the interconversion of states of matter
- Change in temperature
- Change in pressure
Differentiate between solidification and condensation.
Answer
The difference between Solidification and condensation is given below:
| Solidification | Condensation |
|---|---|
| The process by which a substance in liquid state changes into a solid state | The process by which a substance in gaseous state changes into its liquid state |
| Example : Conversion of water to ice | Example : Conversion of steam to water |
Differentiate between melting and boiling.
Answer
The difference between Melting and boiling is given below:
| Melting | Boiling |
|---|---|
| The process by which a substance changes from solid state to liquid state | The process by which a substance changes from a liquid state to vapour state on heating |
| Example : Ice to water | Example : water to steam at 100°C |
Differentiate between gas and vapour.
Answer
The difference between gas and vapour is given below:
| Gas | Vapour |
|---|---|
| A state of matter that stays in the gas form under normal conditions | The gaseous form of a substance that is usually a liquid or solid at room temperature |
| Example : oxygen, nitrogen | Example : water vapour |
Differentiate between miscible and immiscible liquids.
Answer
The difference between miscible and immiscible liquids is given below:
| Miscible | Immiscible |
|---|---|
| Liquids which mix with each other | Liquids which do not mix with each other |
| Example : Water and alcohol | Example : Water and oil |
Differentiate between vaporisation and evaporation.
Answer
The difference between vaporisation and evaporation is given below:
| Vaporisation | Evaporation |
|---|---|
| The process by which a substance changes from a liquid state to vapour state on heating | The change of state of a liquid into vapour below boiling point |
| It includes boiling and evaporation | It a slow form of vaporisation at surface level |
| Example : Water to water vapour | Example : Drying of wet clothes |
State the three effects of heat on matter.
Answer
Change (interconversion) in states of matter — The process by which matter changes from one state to another and back to the original state, without any change in its chemical composition is called interconversion of states of matter.
Thermal expansion of the matter — All the three states of matter, i.e. solid, liquid and gas expand on heating. i.e. they increase in size, without changing their composition. This process is called thermal expansion.
Chemical change — A chemical change is where a substance is transformed into a new substance with different properties.
How is interconversion of states of matter different from a chemical reaction ?
Answer
During the interconversion of state of matter the physical state changes without any change in its chemical composition. Whereas, in a chemical reaction a substance is transformed into a new substance with different properties.
How does a liquid change into its gaseous state? Explain in brief.
Answer
A liquid change into its gaseous state by the process of boiling or vaporisation.
As a liquid is heated, its particles start gaining energy and move more vigorously which increases the gaps between the particles and decrease the force of attraction. Ultimately, a liquid changes into gaseous state.
What happens to a metal ball when it is heated? What does this show ?
Answer
When we heat a metal ball for about 5-6 minutes, it expands. This shows that a solid substance expands on heating. When we cool a solid substance, it again contracts to its original size.
Why does a candle become smaller on burning with time?
Answer
When a candle is lit, first, candle wax melts, then turns into vapour.The vapours react with air to produce two new substances, carbon dioxide and water.
Therefore, a candle on burning becomes smaller and smaller and the part of wax which has undergone chemical change cannot be recovered.
State the two characteristics of a chemical change.
Answer
Two characteristics of a chemical change are.
- It is is permanent change in which new substances are formed from the original substances.
- The properties of the new substances are entirely different from those of the original substances.
Look at the diagram given alongside and answer the questions that follow:
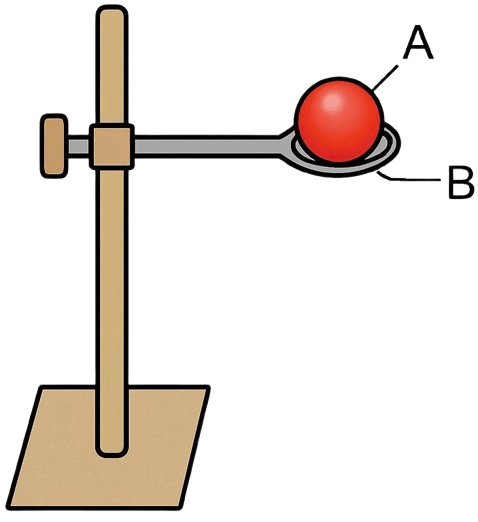
(a) Label A and B.
(b) What is the purpose of this experiment?
Answer
(a) A — Metal ring
B — Metal ball
(b) The purpose of this experiment was to show that a solid expands on heating and when it is cooled back down, it contracts back to its original size.
Given below is a crossword puzzle based on this lesson. Read the clues across and clues downwards and fill up the blank squares.
Across:
- Quantity of matter
- Solid changes directly into vapour
- Gas changes into liquid
Down:
- Occupies space and has mass
- Liquid is heated to change into vapour
- Solid changes into liquid.
Answer
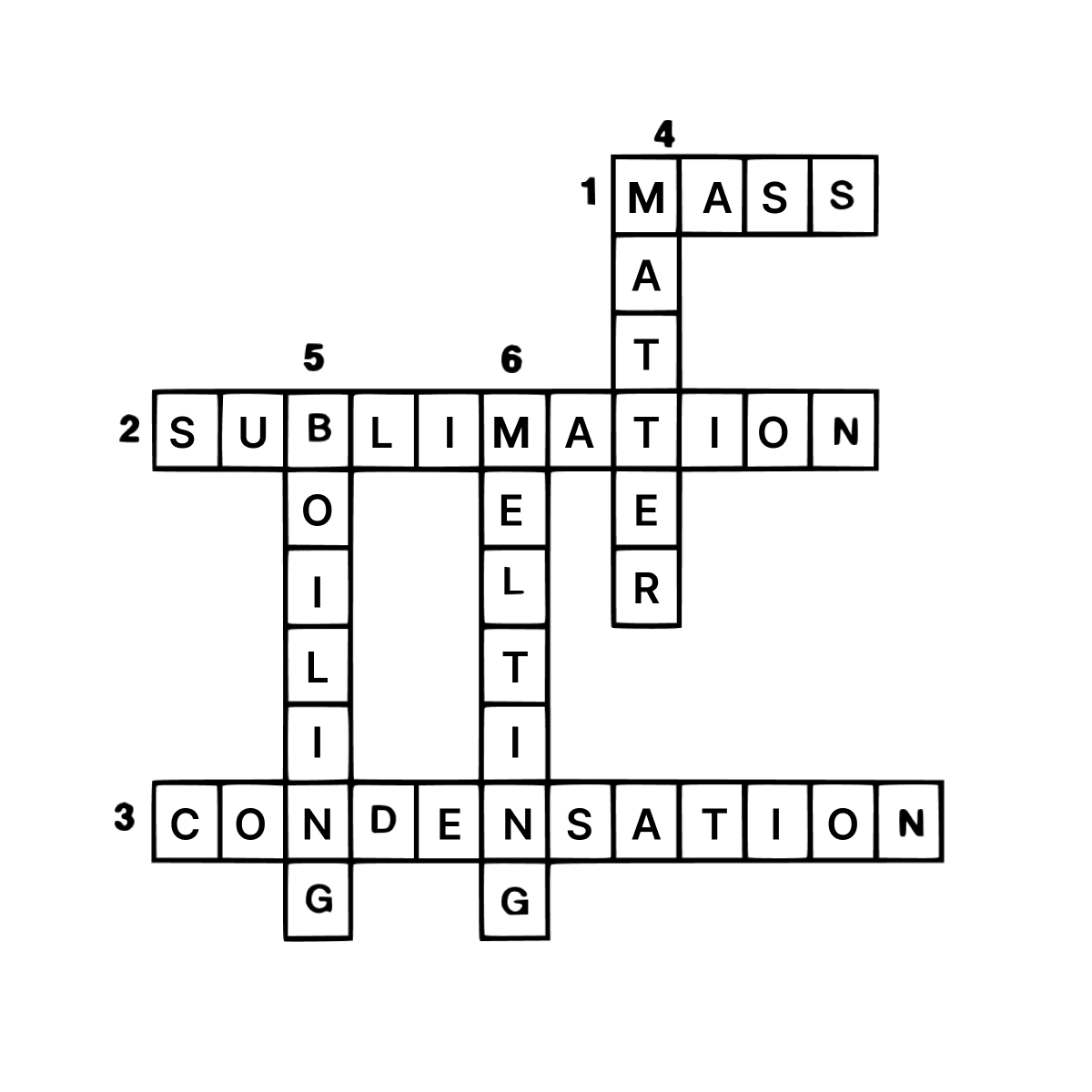
Across:
- Mass
- Sublimation
- Condensation
Down:
- Matter
- Boiling
- Melting